My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.30 no.1 La Habana Jan.-Mar. 2013
RESEARCH
Viral inactivation capacity of Melagenina® Plus during the storage step
Propiedad de inactivación viral durante la obtención de la Melagenina® Plus en la etapa de almacenamiento
Leonor I Lobaina1, Enrique Noa1, Ana M Hernández2, Marta Dubed1, Leonor M Navea1, José R Pérez2
1 Laboratorios de Investigaciones del Sida, Lisida. Carretera de Tapaste y Autopista Nacional, San José de las Lajas, CP 32700, Mayabeque, Cuba.
2 Planta de Derivados de la Placenta, Centro de Histoterapia Placentaria. Carretera de la Autopista Novia del Mediodía y 173, Valle Grande, La Lisa, La Habana, Cuba.
ABSTRACT
This work was aimed at validating the viral inactivation property during the storage step of the production process of Melagenina® Plus, which is a Cuban biological product for the treatment of vitiligo. The product was challenged at storage with high loads of four viral models, corresponding to three enveloped and one non-enveloped virus, two of them RNA and the other two DNA viruses: the human immunodeficiency virus type 1 (HIV-1), the bovine viral diarrhea virus (BVDV), the porcine herpes virus type 1 (PHV-1) and the canine parvovirus (CPV). The viral titer was determined using the Reed-Muench method based on the viral cytopathic effect, and reduction factors were calculated as the difference of viral loads at the beginning and the end of the step. Enveloped viruses were inactivated between days 1 to 3, and the enveloped virus (CPV) was achieved after 21 days. The viral load showed a very highly significant decrease (p < 0.0001), being conditioned to storage temperature (30 ± 5 °C) and ethanol concentration (71 % minimum). The reduction factors achieved on this step (1:5.0 log for HIV-1; 3.5 log for BVDV; 4.24 log for PHV-1 and 5.8 log for CPV) characterized the adequate level of safety of the Melagenina® Plus production process.
Keywords: Melagenina Plus, storage, inactivation, reduction factors.
RESUMEN
Este artículo presenta la validación de la propiedad de inactivación viral de la Melagenina® Plus en la etapa de almacenamiento: producto biológico cubano para el tratamiento del vitiligo. La etapa se retó con altas cargas virales de cuatro modelos virales de tres virus envueltos y uno no envuelto, y de genoma ARN (dos) o ADN (dos), respectivamente: virus de la inmunodeficiencia humana tipo 1 (VIH-1), virus de la diarrea viral bovina (VDVB), virus del herpes porcino tipo 1 (VHP-1) y parvovirus canino (PVC). El título viral se determinó por el método de Reed y Muench basado en el efecto citopático viral; y los factores de reducción se calcularon por la diferencia de la carga viral al inicio y al final de la etapa. La inactivación de los virus envueltos se logró entre el primero y el tercer día, y la inactivación del modelo de virus no envuelto (PVC), a los 21 días. La disminución de la carga viral fue altamente significativa (p < 0.0001), determinada por la temperatura de almacenamiento (30 ± 5 °C) y la concentración de alcohol (mínima: 71 %). Los factores de reducción alcanzados en esta etapa para los modelos virales (VIH-1: 5.0 log; VDVB: 3.5 log; VHP-1: 4.24 log; PVC: 5.8 log) le confieren un adecuado nivel de seguridad al proceso de producción de la Melagenina® Plus.
Palabras clave: Melagenina Plus, almacenamiento, inactivación, factor de reducción.
INTRODUCTION
Human placenta is an organ enriched on active and safe biological substances which are used to produce medicines and cosmetics. One of them is Melagenina® Plus, an internationally reknown pharmaceutical product used for the treatment of vitiligo, which is a dermatological disease affecting 1 % of the world population [1, 2].
Its biological origin forces the product to fulfill both national and international regulatory standards, (in Cuba from the State Center for Medicines Control, CECMED), and also suffice patients’ needs.
Those regulations begin with the control during selection and testing of human placenta as raw material. This organ could be naturally infected by a wide range of DNA or RNA viruses [3-5], with an underlying risk for infectious agents transmission.
In order to circumvent limitations inherent to studying placenta (having to evaluate a wide range of viral contaminants, the lag period of viral infections and the lower sensitivity assay levels required), the viral clearance capacity of the given production process needs to be evaluated. This guarantees the pharmaceutical’s safety and diminishes chances for any virus from placenta (known or undiscovered, unex-pectedly found or dangerous) to remain in the final formulation, being removed or inactivated [6, 7].
In the case of Melagenina® Plus, it is generated by adding calcium to the active pharmaceutical ingredient (API) of Melagenina® lotion. Previously, validation of the Melagenina® lotion manufacturing process demonstrated that this placental extract’s safety was conditioned to its storage in ethanol (71 % or higher) and at room temperature (30 ± 5 °C) [8].
Considering that the production processes of these two human placental extracts only differ in the addition of calcium to the API, this work was aimed at verifying to what extent this change would impact the safety of the Melagenina® Plus regarding viral inactivation during the storage step.
MATERIALS AND METHODS
This research was performed in the AIDS Research laboratory (Lisida, Cuba), equipped with the necessary resources for the inverse scaling of the process and its validation. There were the proper qualified personnel from the Placenta Derivatives Plant (Deplacén, Cuba), and procedures complied with the standards recommended by the national and international regulatory agencies [3, 7, 9].
Sampling
Two lots of Melagenina® Plus were used in the study and one with the Melagenina® lotion as control. Class A ethanol (95 %) and calcium chloride dihydrate were purchased from Quimivita (Quimivita, S.A., Spain), and certified as released by the Quality Assurance Laboratory at Deplacén.
Simulated challenge study
Three flasks of each Melagenina® Plus and the Melagenina® lotion lots included in the study were taken. For each product, one flask was provided with RPMI-1640 culture medium supplemented with 10 % fetal bovine serum (FBS; 1:5 v/v), another with medium supplemented with FBS (1:10 v/v) and the third remained as control. All the samples were assayed in triplicates, identified with a code and further delivered to the Chemical Control Laboratory of the Quality Assurance Vice-Direction at Deplacén, for chemical, microbiological and biological control assays as established for industrial scale productions.
The melanocytopoietic activity was determined by the methodology described by Martínez et al. [10]. Groups of three C57BL/6 male mice, with a weight of 20 to 22 g, were treated topically in the ears for five consecutive days. They received either lot of the product previously exposed to the treatments described, or the placebo (excipient without API). At 72 h of the last application, animals were sacrificed, ears epidermis samples were taken and processed by the L-Dopa histochemistry technique.
Viral models
Viral models were selected according to its similarity with the possible viral contaminants in the placentas, resistance to physical and chemical agents, and its relevance for being transmitted by blood and its derivatives. They comprised enveloped and non-enveloped viruses, either RNA or DNA genome, respectively: the human immunodeficiency virus type 1 (HIV-1), the bovine viral diarrhea virus (BVDV), the porcine herpes virus type 1 (PHV-1) and the canine parvovirus (CPV) (Table 1).
The high-titer control strains were produced from the controlled cell lines at Lisida, and using culture media manufactured and released by the Cell Culture Laboratory at Lisida (Table 1). Each viral strain was amplified into its cellular specific substrate at a multiplicity of infection of 0.3.
Viral inoculums were titrated by the microtitration method in 96-well plates, according to Johnson and Byington [11]. The median tissue culture infective dose (TCID50/mL) was determined as the cytopathic effect, according to the Reed and Muench method [12]. For the HIV-1, the p24 antigen was quantified using the DAVIH Ag p24 kit (Davihlab, Cuba).
Cytotoxicity study
The Melagenina® Plus, Melagenina® lotion, and the purified water solutions of 71 % alcohol and 1 mg/mL CaCl2 were treated in triplicates to decrease its cytotoxicity on each cellular substrate. One volume of each sample was diluted in two and four volumes of a buffered salt solution (BSS), pH 7.2. Serial 1/4 dilutions were made, further adding the cellular substrates at the given concentrations: 5 × 105 cells/mL for the cell line MT4, and 2 × 105 cells/mL for the other cellular substrates). Dilutions were incubated at 37 ºC in a wet atmosphere of 5 % CO2. Four days later, cultures were observed under an inverted microscope and the lowest toxic dilution of samples were determined, compared to the cell culture controls without samples. The dilution showing moderate to minimum cytotoxicity was set as working dilution for sample processing.
Interference study
The viral models were added to citotoxicity samples at the working dilution, and cells were further homogenized. Two samples of each were taken, one after mixing (M1) and a second (M2) after incubation for 1 h at room temperature. A control sample of each viral inoculum was included, being diluted in a supplemented medium and subjected to the same treatment. The assay was made in triplicates.
Subsequently, samples were processed according to results of the cytotoxicity study, with serial dilutions similar to those used to titrate viral inoculums. Viral interference was set as the viral titer reduced more than two logs.
Inactivation during storage of Melagenina® Plus
For the challenge at the storage step, a flask from each selected Melagenina® Plus lot was selected. These represents the 0.03 % of the flasks conforming an industrial lot, which were infected with the viral models at a proportion of 1:5 or 1:10 (v/v) according to the simulated challenge study results. Immediately after the addition of each viral model to the flask, a sample was taken, considering it as the initial viral load (VLi), and flasks were stored at room temperature.
The inactivation kinetics was determined for enveloped viruses monitoring on days 1, 2, 3 and 7 of storage. The VL assessed on day seven was considered as the final VL (VLf). The study was carried out in triplicates.
The following controls were included:
- Viral strain in 1 mg/mL CaCl2 and in 71 % ethanol, both prepared with purified water.
- Viral strain in supplemented culture media (stored at room temperature, 4 and -85 ºC).
- A flask of Melagenina® lotion, to check if the precipitate observed in the Melagenina® Plus once added the CaCl2 had any incidence in the inactivation property at storage.
Control samples were kept at the same storage temperature of Melagenina® Plus and VLs were quantified at the same time intervals.
Reduction factor calculation
Reduction factor (RF) at storage was calculated according to the following equation [9, 12]:
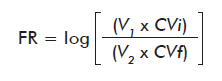
Where:
V1 and VLi: are the initial volume and viral load of the sample; V2 and VLf: are the final volume and viral load of the sample.
The process step was classified as effective, moderately effective or ineffective, according to the viral reduction logarithms guidance of the Committee for Proprietary Medicinal Products [9].
Statistical analysis
Viral challenge results were analyzed with the aid of the Statgraphics Plus program, (Statpoint Technologies, Inc., USA; version 5.0, 2000). A Student’s t-test was used for paired data, to identify statistically significant differences between VLi and VL values at different time intervals for each viral model.
RESULTS AND DISCUSSION
Simulated challenge
Addition of the supplemented media, at a proportion of 1:5 (v/v), to the Melagenina® Plus and the Melagenina® lotion, significantly modified (p < 0.01) several quality parameters of these products, such as: ethanol concentration, absorption, cholesterol, nitrogen and proteins concentrations. Therefore, they were not accepted, and the viral challenge was not carried out with that proportion of supplemented media.
When the medium was added at half the proportion 1:10 (v/v), some physicochemical parameters were altered, but not significantly: absorption, evaporation residues, cholesterol, nitrogen and protein concentrations (Table 2). Lipids concentration remained within the established acceptance limits. This parameter was regarded as essential for the melanocytopoyetic activity of placental extracts [2, 13].
Melagenina® Plus diluted 1:10 (v/v) in culture medium showed no significantly different biological activity in mice melanocytes, compared to animals treated either with Melagenina® or the control lot of the product (Table 2). Therefore, these results were accepted, and the 1:10 (v/v) medium proportion was set for viral challenge at storage.
Key aspects are considered in the viral validation study: the proper design of the production process at laboratory scale and results from the simulated challenge, to demonstrate the influence of the culture media used to dilute infectious agents on the physicochemical and biological parameters established for the product [14-16].
Cytotoxicity study
Samples’ cytotoxicity were mild to moderate (Table 3), with samples diluted 1:2 (v/v) in BSS showing mild to moderate cytotoxicity, while those diluted 1:5 (v/v) showed minimal changes at best in cell cultures. No differences were observed neither between the two lots of Melagenina® Plus nor of any of them to the one of Melagenina® lotion. Such results were determinant for the selection of the 1:2 (v/v) BSS dilution as the procedure to decrease samples’ cytotoxicity.
Several methods have been reported to decrease cytotoxicity (dialysis, gel filtration chromatography, precipitation and serial dilutions); but either the method, special care must be taken to do not deteriorate viral titers, which is detrimental for detecting low VLs [9, 15, 16].
Viral interference study
Viral interference was determined by decreasing or not of the viral models titer in samples, in comparison with the control models (Table 4). The titer decrease for viral controls diluted 1:10 (v/v) in supplemented culture medium did not achieved 1 log.
When adding the enveloped virus models to placental extracts, a fast decrease in viral titers of 2 or more logs was observed, significantly different from the viral control (p < 0.05). After 1 hour of incubation, baseline VLs were detected, with highly significant differences (p < 0.01). A very similar behavior was observed for the viral control dissolved in 71 % ethanol solution (Table 4). No significant differences were detected between CPV viral titers, regardless ethanol.
These results are in agreement with those from other groups, reporting a marked susceptibility of enveloped viruses to lipidic solvents, and particularly to alcohol concentrations higher than 70 %, but not of the non-enveloped viruses [6, 17, 18].
These results demonstrate that placental extracts do not interfere in the replication of these viral models. The viral interference study provided evidences on the action of the raw materials and the biological products over the viral models’ titers, as well as the selection of adequate viral models to study the viral clearance capacity of the Melagenina® Plus production process.
Viral inactivation capacity of Melagenina® Plus during storage
The behavior of enveloped model viruses during the storage of Melagenina® Plus are shown in figure 1. There were no statistically significant differences among viral titers for both lots of Melagenina® Plus and the controls used in the study (Melagenina® lotion and 71 % ethanol solution). Significant differences (p < 0.05) were found in VLi for samples containing 71 % ethanol compared to viral controls, in agreement with results from the viral interference study.
The three viral models followed the typical viral behavior seen for viruses of low resistance to organic solvents, characterized by a sharp drop of titers and complete inactivation within a short period. This last property is related to their relative ethanol resistance. In all the cases, very highly significant differences were detected between VLi and VL assessed after 24 h (p < 0.001) (Figure 1).
PHV-1 was completely inactivated in both infected placenta extracts during the first 24 h of storage, and also in the 71 % alcohol solution (Figure 1). RNA viruses showed a more delayed inactivation, for HIV-1 achieved after 24-48 h of storage with the Melagenina® lotion and 48-72 h after storage with both lots of Melagenina® Plus.
BVDV showed an intermediate inactivation progression, achieved within the first 24 h of storage with the Melagenina® Plus and after 48 h of storage in Melagenina® lotion and 71 % alcohol. None of the three enveloped virus models showed significant differences in VL compared to controls at any time points.
In the case of BVDV, it is affected by lipid solvents and chemical detergents, but tends to stabilize with the addition of proteins [15, 19]. Nevertheless, the BVDV inactivation observed was not related to the amount of lipid precipitates in the placental extracts, due to the short storage periods assayed.
There were differences in the titers of enveloped virus controls stored at different temperatures. Viral titers decreased in those stored at room temperature, but not at 4 or -85 ºC (Figure 1). For HIV-1, viral titer showed statistically significant differences (p < 0.05) only after seven days of storage at room temperature, compared to the titer of the viral control stored at 4 ºC. Such differences were detected for PHV-1 since the third day (p < 0.05) and maintained until the seventh. Differences were detected earlier for BVDV, starting at 24 h after storage at room temperature (p < 0.05), and becoming highly significant at 48 h (p < 0.01) and sustained until day seven.
Similar results were reported by Ruibal et al. [20], while validating an intravenous immunoglobulin production process. They observed a drop in BVDV titers below 6 logs when stored at 21 ºC for 21 days, while storage at 4 ºC did not affect titers.
The decrease of viral titers for controls stored at room vs. low temperature demonstrated that room temperature storage reinforces the virus inactivation capacity of the product.
The inactivation kinetics of viral models in CaCl2 did not differed from the viral control at room temperature, except for the statistically significant differences (p < 0.05) detected for PHV-1 (Figure 1). This indicates that the drop in viral titers was caused by the storage temperature.
The inactivation kinetics for the non-enveloped virus model (CPV) is shown in figure 2. After 24 h of storage, a highly significant drop in viral titers was detected for all the samples in 71 % alcohol, compared to the respective VLi values. The VL values stabilized borderline to the detection limit of the assay after 48 h, and remained steady until day seven, with very highly significant differences (p < 0.001). This viral model was fully inactivated in the Melagenina® lotion on day 14, and after 21 days in both lots of Melagenina® Plus and 71 % alcohol (Figure 2).
Titers of controls stored at 4 and -85 °C remained as initially determined during the whole experiment. They dropped for the room temperature storage since day seven, becoming progressively significant on day 14, highly significant on day 21 and very highly significant on day 28 (p < 0.001), this last accounting for a 5.41 logs decrease.
The CPV inactivation kinetics in 1 mg/mL CaCl2 behaved very similar to the viral control stored at room temperature, evidencing the lack of inactivating capacity for CaCl2 at the assayed concentration and reinforcing the conclusion that temperature was the key parameter leading to virus inactivation.
All these results corroborate the effectiveness of the experimental design to study the capacity for viral inactivation during storage of the production process of the placental extract Melagenina®. In fact, enveloped viruses were inactivated within a short period (24 h), with the non-enveloped CPV showing a marginal VL at 72 h of storage [7].
Since CPV, a highly resistant virus, was completely inactivated upon storage, safety was increased both for Melagenina® Plus and its production process. Therefore, CPV results could not be considered relevant just for the non-enveloped viruses, but also this virus can be regarded as a non-specific viral model which provides evidences for the inactivation of new or unpredicted viral contamination in raw materials [7, 9, 15].
Table 5 shows RFs of both placental extracts upon storage, and their respective times for inactivating the challenge VLs. Consequently, enveloped virus were quickly inactivated, after three and four days of storage for Melagenina® Plus and Melagenina® lotion, respectively, without significant differences. The non-enveloped viral model was inactivated after 14 and 21 days of storage for Melagenina® and Melagenina® Plus, respectively, with very highly significant differences (p < 0.001) for each placental extract.
Very highly significant differences (p < 0.0001) were observed between the times for inactivation of enveloped and non-enveloped viruses. The difference on time required for viral inactivation in both placental extracts of Melagenina® Plus was related to the presence of greater amounts of lipid precipitates, which could form lattices that protect viruses from the action of the inactivating agent, and, therefore, increase the time required for full inactivation.
Very similar results were observed when validating the placental extract EP-100 with the enveloped virus models, but not for the non-enveloped viruses. These last required a longer storage period (63 days) for complete inactivation [21]. It was related to the storage temperature (4 ºC) and the ethanol concentration in the EP-100 extract. That results corroborate that these two factors are fundamental for the safety of the Melagenina® Plus placental extract.
FRs higher than 4 logs were attained except for the BVDV, and the four viral models were effectively inactivated during the storage step of Melagenina® Plus. The challenge VLs of either enveloped or non-enveloped viruses were fully inactivated, regardless variations in the parameters for this step (Table 5).
The low resistance of the enveloped model viruses to ethanol at the different steps matches that of viral validation studies of different processes for biologicals’ production, being closely related to the conditions for the action of the inactivating agent (medium properties, temperature, humidity and pH) [7, 22, 23].
The virucidal action of ethanol is affected by low temperature and high protein and lipid concentrations, these factors stabilizing viruses and making them more resistant to the adverse conditions of the culture medium and disinfectants [7, 18, 22, 23]. Both culture medium and ethanol were absent during placental extract storage, favoring the inactivation of all the viral models while validating the production process.
In summary, the Melagenina® Plus formulation in alcohol solution of 71 % or higher, together with its storage at room temperature, supported the complete inactivation of all the viral models tested, regardless its resistance to inactivating agents. It provided an adequate safety level to the production process, being able of eliminating new or unpredictable viral contaminations of placentas.
REFERENCES
1. Miyares C. Melagenina® Plus. Avances Médicos en Cuba. 2000;7(23):50-2.
2. Miyares CM, inventor; Centro de Histoterapia Placentaria, assignee. Composition for stimulating the synthesis of the melanic pigment and process for obtaining it. United States patent US 6660305. 2003 Dec 9.
3. Centro para el control estatal y la calidad de los medicamentos. Regulación No. 2/2002. Placenta humana como materia prima farmacéutica. La Habana: Cecmed; 2002.
4. Chow SS, Craig ME, Jacques CF, Hall B, Catteau J, Munro SC, et al. Correlates of placental infection with cytomegalovirus, parvovirus B19 or human herpes virus 7. J Med Virol. 2006;78(6):747-56.
5. Indolfi G, Moriondo M, Galli L, Azzari C, Poggi GM, Resti M, et al. Mother-to-infant transmission of multiple blood-borne viral infections from multi-infected mothers. J Med Virol. 2007;79(6):743-7.
6. Barin F. La sécurité virale des médicaments d’origine biologique. Ann Pharm Fr. 2008;66(3):129-39.
7. Food and Drug Administration. Current good manufacturing practice for finished pharmaceuticals. Subpart E- Control of Components and Drug Product Containers and Closures § 211.80 General requirements (21 CFR 211.80). Rockville MD: Food and Drug Administration. Department of Health and Human Services; 2008.
8. Noa E, Hernández AM, Ruibal I, Dubed M, Navea L, Lobaina L, et al. Validación de la capacidad de inactivación viral del proceso de producción del extracto placentario loción Melagenina®. Rev Cub Farmacia. 2002;36(Suppl 1).
9. Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Virus validation studies: The design, contribution and interpretation of studies validating the inactivation and removal of viruses. London: The European Agency for the Evaluation of Medicinal Products, Human Medicines Evaluation Unit; 1996 Feb. CPMP/BWP/269/95. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003684.pdf.
10. Martínez L, Sanz A, Miyares CM, Hollands I, Roque S, Pimienta R, et al. Estudio comparativo del efecto pigmentante de dos extractos de placenta humana (Melagenina I y II). In: La histoterapia placentaria en algunas afecciones dermatológicas. La Habana: Palacio de las Convenciones; 1994.
11. Johnson VA, Byington RE. Infectivity assay (virus yield assay) In: Aldovani A, Walker BD, editors. Techniques in HIV Research. New York: Stockton Press; 1990. p. 71-6.
12. Reed LJ, Muench H. A simple method of estimating fifty percent end point. Am J Hyg. 1938;27(3):493-7.
13. González Y, Salcedo G, García W, Hernández CR, Brito N. Validación de la técnica espectrofotométrica de cuantificación de lípidos totales para el control de la calidad de los extractos de placenta. In: Memorias II Seminario Internacional de Histoterapia Placentaria. La Habana: Centro de Histoterapia Placentaria; 2006.
14. Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Plasma-Derived Medicinal Products. London: The European Agency for the Evaluation of Medicinal Product, Evaluation of Medicines for Human Use; 2001 Jan. CPMP/BWP/269/95 rev. 3. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003613.pdf.
15. Darling A. Validation of biopharmaceutical purification processes for virus clearance evaluation. Mol Biotechnol. 2002;21(1):57-83.
16. Tuñón MA, Noa E, Sánchez K, Ruibal IJ, Dubed M, Castañeda F, et al. Desescalado del proceso de producción de Hebertrans para su validación viral. Biotecnol Apl. 2004;21:229-33.
17. Azzi A, Maggi F, Zakrzewska K, Menconi MC, Di Pietro N, Salotti V, et al. Different behavior of erythrovirus B19 and torquetenovirus in response to a single step of albumin purification. Transfusion. 2006;46(7):1162-7.
18. Suchomel M, Gnant G, Weinlich M, Rotter M. Surgical hand disinfection using alcohol: the effects of alcohol type, mode and duration of application. J Hosp Infect. 2009;71(3):228-33.
19. Rivera H. Evolución del conocimiento sobre la enfermedad de la diarrea viral bovina y su agente etiológico. Rev Investig Vet Perú. 2008;19(2):93-112.
20. Ruibal Brunet IJ, Noa Romero E, Rivero Mas AT, Martín García RZ. Inactivación del VDVB (modelo experimental del virus de la hepatitis C) por pH bajo y tratamiento por calor en inmunoglobulinas humanas endovenosa. Sangre (Barc). 1999;44(5):352-6.
21. Pérez JR, Noa E, Vergel V, Alfaro E, Lovaina L, Sánchez Y, et al. Viral cleaning capacity of the manufacturing process of the EP-100. Avances en Biotecnología Moderna. 2007:CP-43.
22. Dichtelmûller HO, Germer M, Rudnick DC. A general approach to robustness studies for virus inactivating and partitioning steps used in production of plasma derivatives. Bioprocess Int. 2005;3(Suppl 7):35-8.
23. Brorson K. Advances in viral clearance. In:. Shukla AA, Etzel MR, Gadam S, editors. Process scale bioseparations for the biopharmaceutical industry. Boca Raton: CRC Press, Taylor & Francis Group. 2007; p. 449-62.
Received in July, 2010.
Accepted in July, 2012.
Leonor I Lobaina. Laboratorios de Investigaciones del Sida, Lisida. Carretera de Tapaste y Autopista Nacional, San José de las Lajas, CP 32700, Mayabeque, Cuba. E-mail: lisida@infomed.sld.cu.













