My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.31 no.2 La Habana Apr.-June 2014
FOCUS
Nimotuzumab, effective immunotherapy for the treatment of malignant epithelial tumors
Nimotuzumab, inmunoterapia eficaz para el tratamiento de tumores epiteliales malignos
Giselle Saurez-Martínez, Anamary Bencomo-Yanes
Departamento de Gerencia Médica y Asuntos Regulatorios, Centro de Inmunología Molecular, CIM. Calle 206 No. 1926 e/ 19 y 21, Atabey, Playa, CP 11600, La Habana, Cuba.
ABSTRACT
Nimotuzumab, a humanized monoclonal antibody, is a novel drug against the epidermal growth factor receptor, which is a protein that is highly expressed in malignant tumors of epithelial origin. This paper presents its physicochemical and pharmaceutical characteristics, the results of pre-clinical and clinical research, and the international regulations for the diseases for which its use has been indicated: in advanced head and neck tumors, malignant brain tumors in adults and children, and advanced esophagus tumors. Its safety profile, efficacy and effectiveness studied before and after its regulatory approval are also described. Finally, recommendations are given for its dosage according to clinical evidence, for the appropriate therapeutic use of this medication.
Keywords: epidermal growth factor receptor, nimotuzumab, cancer treatment, targeted therapy, biological therapy.
RESUMEN
Nimotuzumab, anticuerpo monoclonal humanizado, es un novedoso fármaco contra el receptor del factor de crecimiento epidérmico: proteína altamente expresada en tumores malignos de origen epitelial. En este artículo se exponen sus características físico-químicas y farmacéuticas, los resultados de investigaciones preclínicas y clínicas, y las regulaciones internacionales para las enfermedades en las que se ha indicado su uso: en tumores avanzados de cabeza y cuello, tumores cerebrales malignos en adultos y niños, y tumores avanzados de esófago. Se describen su perfil de seguridad, eficacia y efectividad, antes y después de su aprobación. Por último se ofrecen recomendaciones posológicas según las evidencias clínicas, para el uso terapéutico adecuado de este medicamento.
Palabras clave: receptor del factor de crecimiento epidérmico, nimotuzumab, tratamiento de cáncer, terapia blanco, terapia biológica.
INTRODUCTION
The receptor of the epidermal growth factor (EGFR) is a transmembrane protein related to proliferation and maturation of cells that are basically of epithelial origin: skin, intestinal mucosa and the liver [1]. Essentially, its activation depends on two ligands: the epidermal growth factor (EGF) and the tumor growth factor alpha (TGF-α), leading to the activation of the signaling cascade of the EGF-EGFR system after phosphorylation and the dimeric formation of that receptor.
This system has been widely studied and is very attractive as a tumoral therapeutic target. It is associated to anarchical proliferation, cellular immortalization, inhibition of apoptosis, neo-angiogenesis and metastasis; all signs of a bad prognosis that provokes resistance to conventional oncologic treatments, such as radiotherapy, chemotherapy and hormonal therapy [2-4].
Passive immunotherapy with MAbs is one of the most effective treatments against the EGFR [5]. There are currently several MAbs against this receptor that have been registered for the treatment of some solid epithelial tumors: Cetuximab® (chimeric MAb), nimotuzumab (humanized MAb) and Panitumumab® (human MAb) [6].
Nimotuzumab is an international generic denomination that is alternatively known worldwide by the brands Theraloc® (registered trademark for the European Union), TheraCIM® (registered trademark for Canada, Indonesia and other Asian countries), CIMAher® (registered trademark for Cuba and Latin America) and BIOMAb-EGFR® (registered trademark of the product produced in India).
This paper summarizes its pharmacotherapeutic characteristics as an anti-EGFR agent, as well as the most recent pre-clinical and clinical evidence endorsing its use.
MECHANISMS OF ACTION AND CLINICAL PHARMACOLOGY
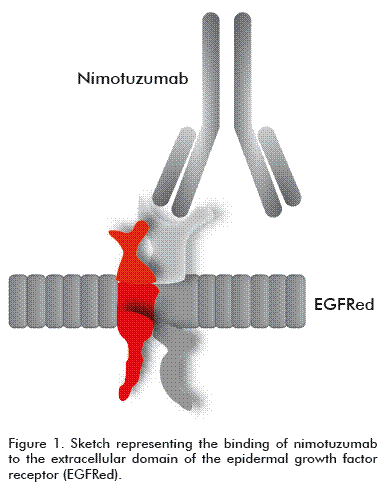
Nimotuzumab is a humanized MAb, an IgG1 isotype immunoglobulin obtained by recombinant DNA technology and produced in mammalian cell lines (murine myeloma NSO) [7, 8]. It recognizes the EGFR with an intermediate affinity of 10-9 M [7]; it contains the hypervariable regions (CDR) of murine origin (ior egf/r3) and the frames of the variable regions and of the constant regions of the heavy and light chains of human origin (Figure 1) [7].
Its binding will block the interaction of the two main ligands of the EGFR: the EGF and the TGF-α. This inhibits the tyrosine kinase activity of the receptor and arrests the cellular cycle at phase G1-S, with a marked anti-proliferative effect [7, 9, 10]. Moreover, it exerts anti-angiogenic and pro-apoptotic effects in tumors that over-express EGFR (Figure 2) [9-12], and reduces the number of CD133+ cells (tumor stem cells in charge of radio-resistance) [13].
PHARMACODYNAMICS
Several in vitro and in vivo studies demonstrated that nimotuzumab inhibits growth and survival of tumor cells expressing EGFR [9, 12, 14]. In clinical trials it was also observed that it prevents phosphorylation of EGFR and the activation of the ERK protein (MAPK), and reduces tumor cell proliferation: a more significant effect in tumor tissues over expressing the EGFR in the skin [15-17]. Other clinical findings demonstrate the synergy of this MAb when combined with radiotherapy and chemotherapy [10, 16, 18-22].
PHARMACOKINETICS
Pharmacokinetics covers processes related to absorption, biodistribution, biotransformation and the elimination of drugs in the body. In this sense, the half life period of nimotuzumab, administered in doses of 50 and 100 mg, ranged between 2 and 3 days; while in larger doses of 200 and 400 mg it lasted longer, ranging between 10 and 14 days (Table 1) [23].
With greater volumes of the MAb there was an increase in the half life of its distribution, elimination and of the distribution volume of the steady state. At the same time, growing concentrations of the MAb provoked a decrease of the plasmatic clearance values, up to the dose level of 200 mg, which corresponds to non-linear pharmacokinetics [23].
In most of the pediatric patient populations evaluated, the maximum half life period was obtained as of the third dose, on increasing the maximum concentration after many dosages, compared to a first administration in which there was no variation of the half life period. The plasmatic clearance and the distribution volume in the steady state decreased after multiple doses, compared to a single dose [24].
These results led to the proposal that the therapeutic advantage of nimotuzumab, defined as the optimum biological dose, is in the range of 200 to 400 mg for adult patients, and 150 mg/m2 of body surface for pediatric patients. And the MAb application interval should not be of more than 2 weeks [23, 24].
To determine its biodistribution, we made gammagraphic studies using nimotuzumab marked with Technetium-99 (mTc99). As target organs we identified the liver, heart, spleen, kidneys and urinary bladder. There was a significant capture in the liver, while its incorporation into the other organs was from slight to moderate (Figure 3) [10, 23].
CLINICAL EFFICACY
More than 20 clinical trials have concluded for their assessment in several tumors, as proof of concept, efficacy tests and therapeutic efficacy that endorsed their sanitary registration, which include:
- Treatment of head and neck tumors at advanced stages, combined with radiotherapy, chemotherapy or both.
- As a monotherapy for highly malignant astrocytoma in children receiving onco-specific treatments with a refractory response.
- Treatment of Glioblastoma multiforme (GBM) combined with radiotherapy in adult patients.
- Treatment of carriers of non-operable malignant esophagus tumors of epithelial origin combined with chemoradiotherapy (CRT) [25].
The use of nimotuzumab has been evaluated when combined with cytostatics, with alkylating agents (cyclophosphamide, cisplatin, carboplatin), vinca alkaloids (vinblastin, vinorelbin, etoposide), topoisomerase inhibitors (irinotecan), cytostatic antibiotics (adriamycin, mitoxantrone), anti-metabolites (methotrexate, 5-fluorouracil) and taxans (docetaxel), among others [18-20, 22, 24, 26-33]. The patients treated with these combinations have tolerated them very well, without showing exacerbation of adverse reactions to these medications. We must point out that there is no knowledge on nimotuzumab possible antagonic interactions with other medications. Below are details of the main clinical results in the diseases for which it has been registered.
Advanced head and neck tumors
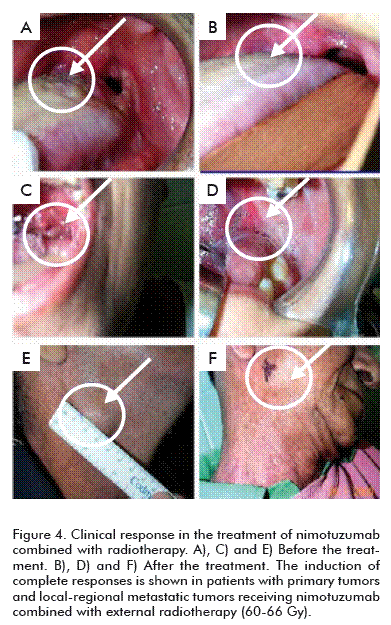
Phase I and II trials to assess the dose and therapeutic effect of nimotuzumab in advanced head and neck tumors, carried out in Cuba and Canada, demonstrated that when it is combined with radiotherapy there is an increase in the percentage of objective response of between 70 and 100 %, compared to the objective response of the radioactive therapy, which is in the range of 30 to 40 % (Figure 4) [10, 34].
To confirm the efficacy of the nimotuzumab in patients with newly diagnosed non-resectable advanced head and neck tumors, three controlled and randomized comparative studies were carried out [16, 18, 35].
An initial trial in Cuba assessed the combination of nimotuzumab with radiotherapy, against radiotherapy plus placebo. The response rate in patients receiving nimotuzumab and radiotherapy was of 59.5 %, which is significantly higher than the value of those receiving the radiotherapy and placebo that was of 34.2 % (Fisher test, p = 0.038). There was a significant increase of survival in the subjects treated with nimotuzumab (median: 12.5 months), compared with the group receiving placebo (median: 9.5 months), according to the Harrington-Fleming statistical test (p < 0.05). In the subgroup of patients with a high expression of EGFR (3+), having the worse prognosis, there was an even greater benefit in those receiving nimotuzumab: 19.6 months of survival, compared to the group receiving radiotherapy alone: 6.4 months (p < 0.05) [16].
A second randomized trial, carried out in India, evaluated the effect of nimotuzumab combined with radiotherapy and CRT. The objective response rate in groups receiving nimotuzumab combined with radiotherapy was of 76 %, and 100 % in those receiving nimotuzumab combined with CRT. In the other two groups of patients receiving radiotherapy or CRT alone, the objective rate of response was of 40 and 76 %, respectively. These differences were statistically significant. The inclusion of MAbs in radiotherapy and in CRT also significantly increased survival, which was estimated at a mean of 49.4 months compared to 16.4 months, in those only receiving radiotherapy and conventional CRT (risk of death, HR: 0.517) [18].
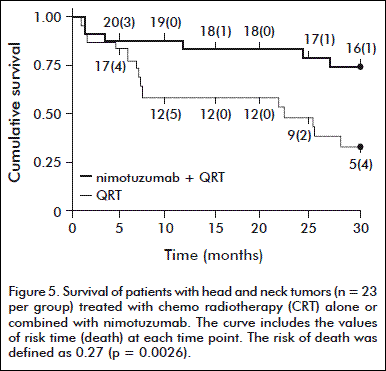
The survival rate at 48 months was significantly higher in the two groups of patients: 47 % in those receiving nimotuzumab and CRT, against 21 % in the group receiving CRT. At the same time, the survival rate in the group receiving nimotuzumab and radiotherapy was of 34 %, compared with the group of patients treated with conventional radiotherapy, which was of 13 % (Figure 5) [36].
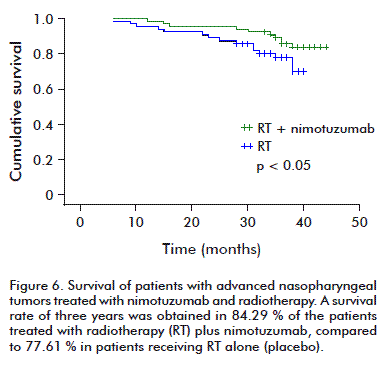
A third trial in patients with advanced nasopharynx tumors, in China, evaluated the use of nimotuzumab combined with radiotherapy. The objective response rate after treating for 17 weeks was of 90.6 %; and it was 51.5 %, in the group treated with radiotherapy alone. The evolution of the patients was later studied, showing that the group of patients receiving the combined treatment of nimotuzumab and radiotherapy had a 3-year survival of 84.3 %; compared to the group treated with radiotherapy alone, in which it was 77.6 % (p < 0.05 %) (Figure 6) [35].
Post-marketing experience in the indication of advanced head and neck tumors
An observational clinical trial was carried out in Cuba to evaluate the combined treatment of nimotuzumab and radiotherapy, and of nimotuzumab and CRT, in patients with advanced head and neck tumors. In this study a survival rate at 48 months of 62 % was estimated [37]. In an analysis of survival according to the number of doses of nimotuzumab received, it was found that the patients receiving the induction treatment alone (6 doses) reached a mean survival of 12.3 months [38]. That time was equivalent to that described in studies before its registration in which an identical therapeutic regime was applied of only 6 doses combined with radiotherapy (12.5 months and 14.4 months) [16, 18]. In patients who continued the monoclonal treatment as maintenance beyond the 6 doses, this median was not achieved [38]. This result serves as the basis for the proposal of the extension of the nimotuzumab treatment as maintenance.
A study made after registration (phase IV), ongoing now in Cuba, assesses the combined therapy of MAb with radiotherapy and chemotherapy, as well as the maintenance therapy. In a partial analysis, the estimation of the median for survival for the nimotuzumab and radiotherapy group was of 14.6 months, while the combination of the MAb together with CRT was of 32.5 months (Piedra P, Nimomeeting 2012; personal communication).
In terms of survival, these studies in an open population confirm the effectiveness of the combination of nimotuzumab with conventional radiotherapy in patients with advanced head and neck tumors and they uphold the clinical benefit of their use in maintenance therapy.
Therapy for high malignancy grade gliomas
The World Health Organization classifies the gliomas of high malignancy grade as anaplastic astrocytomas (AA or degree III) and multiform glioblastomas (GBM or degree IV) [39].
The life expectancy of patients with GBM alone in a support treatment is of 3 months, and the survival rate after one year is of 3 %. The inclusion of radiotherapy after the surgical resection of the tumor increases survival in 8 months, with a rate of survival after one year of 24 % [40].
The proof of concept study, combining nimotuzumab and radiotherapy after surgery, achieved objective responses of 37.9 % in patients and the stabilization of 41.4 %, with an overall survival of 22.17 months. However, in patients who were MGB carriers it was of 17.47 months, and it did not reach the median of those with AA [41]. Three controlled and randomized studies for the evaluation and confirmation of efficacy were later carried out [19, 21, 24].
The first trial (phase II/III) in Cuba, evaluated the combination of nimotuzumab and radiotherapy. Overall survival of 44.5 months was significantly higher in the experimental group compared to 30.4 months in the control group that only received radiotherapy [21]. The group of patients with GBM treated with the MAb and radiotherapy, which followed the treatment schedule programmed in the protocol, doubled the median survival, compared to the control group treated with radiotherapy and placebo, i.e., 16.1 versus 8.6 months. At the same time, the survival rate at two years was higher in patients with GBM or AA treated with nimotuzumab compared to those untreated: 26 versus 0 %, and 72.2 versus 36 %, respectively [21].
As the conventional therapy in patients with de novo GBM is surgery, followed by radiotherapy and chemotherapy with temozolamide (TMZ), we also evaluated the combination of nimotuzumab with radiotherapy and TMZ [19, 24].
One of these studies (phases I/II) in China, described an objective response rate of 70.0 %, in the group receiving nimotuzumab, and of 52.4 % in the control group. The median survival of these groups was 16.5 and 10.5 months, respectively. The survival rate after one year was 81.3 % (for the group receiving nimotuzumab) and 69.1 % (for the control group). Although these differences were not significant, there is a trend toward a better performance of the combination of MAb with conventional therapy [19].
The following study (phase III) was in Germany. The result of survival between groups was of 22.3 months (confidence interval, CI, was 17.2-26.5) in the group receiving nimotuzumab plus radiotherapy and TMZ, and of 19.6 months (CI of 14.8-24.0) in the group receiving radiotherapy and TMZ. An analysis of the poor prognosis factors, where patients are grouped according to whether there was a post-surgery tumor, tumoral necrosis of 10 % and a non-methylated state of the methylguanine methyltransferase enzyme (MGMT), showed a significant difference in survival in favor of patients receiving nimotuzumab compared to the control group (23.8 versus 13.8 months, p = 0.03) [42].
This finding indicated that there is a subpopulation of patients who, in spite of their poor prognosis, even when disfavored with TMZ, can benefit from the combination with this new therapeutic alternative. Therefore, future studies must investigate the efficacy in this type of patient.
Post-marketing experience in the indication of malignant gliomas
To confirm the effectiveness of the combination of nimotuzumab with radiotherapy after surgery in patients with recently diagnosed malignant gliomas (AA and GBM), an observational post-marketing clinical trial was carried out in Cuba. All patients received nimotuzumab at the dose and schedule for induction and maintenance recommended in the leaflet, and they were followed-up for three years. At the end of the study the median survival was estimated in 13.7 months for the patients with MGB, and the median was not reached in patients with AA [37].
A partial analysis of another phase IV trial in progress in Cuba describes a progression-free survival (PFS) rate at 24 months of 18 % in patients with GBM and of 65 % in patients with AA, with a survival rate at 24 months of 17 and 59 %, respectively [43].
All studies carried out before and after the registration of nimotuzumab confirm the therapeutic efficacy of the combination of the MAb with radiotherapy and potentially with CRT. At the same time, there is evidence that the prolonged maintenance with this drug also increases survival when it is used to treat these conditions.
Treatment of pediatric patients with high malignancy grade astrocytomas: recurrent, refractory or both
Life expectancy in pediatric patients with recurrent brain tumors that are refractory to conventional treatments such as surgery, radiation and chemotherapy, is of approximately one month. Besides its bad prognosis, there is no standard treatment for this condition [44].
Taking into account this urgent medical need and the therapeutic potential of nimotuzumab in neuroepithelial tumors, an open uncontrolled clinical trial was carried out in Germany in pediatric patients with high malignancy grade astrocytic tumors that were recurrent or refractory to conventional treatments [24]. For 12 weeks, they received 150 mg of nimotuzumab -per m2 of body surface as a monotherapy, with maintenance every 15 days, left to the discretion of the researcher, in responders. A total of 37.8 % of the patients treated perceived objective responses, particularly in the subgroup of patients with brain stem tumors.
In relation to PFS, a median of 50 days was estimated (one day standard deviation, range: 12-637 days, CI: 48-52 days). The median survival of all patients was of 140 days; approximately 5 months (standard deviation of 18 days, range: 12-1180 days, CI: 104-176 days). It was significantly different between responders and non-responders: 6.1 versus 3.6 months (p = 0.001) [24].
Post-marketing experience in the treatment of pediatric patients with high malignancy grade astrocytomas: recurrent, refractory or showing both types of behavior
As a consequence of the previous study, a program was developed for its expanded clinical use in pediatric patients with recurrent brain tumors, in Cuba. Nimotuzumab was administered in doses of 100 mg and 150 mg/m2 as a monotherapy and combined with chemotherapy. The disease control rate and the survival rate after one year reached 64 %. The most important findings include the recovery of neurologic functions and the improvement of the general condition of the patients during the treatment, especially in those where the disease was controlled [45]. Figure 7 shows the response to the treatment with nimotuzumab in a patient included in this study.
Other studies in pediatric glioma
A recent multinational (Germany, Italy and Russia) phase III uncontrolled clinical trial evaluated the efficacy of the combination of nimotuzumab with radiotherapy in pediatric patients with newly diagnosed diffuse brain stem gliomas. The survival median was of 9.4 months and the PFS rate was of 33.3 %. These results demonstrate that the combination of nimotuzumab with radiotherapy did not surpass the efficacy of chemotherapy in this disease, although nimotuzumab was less toxic [24].
In Italy, we evaluated another therapeutic alternative in pediatric patients with diffuse brain stem gliomas, consisting of the combination of nimotuzumab with radiotherapy and vinorelbin [24, 46]. The PFS rate was of 20 % after one year; the general rate of survival was of 73 %; and of 25 % at one and two years.
In agreement with the latter, it has been demonstrated that nimotuzumab is effective when administered alone and combined with radiotherapy and chemotherapy for newly diagnosed and recurrent pediatric glial tumors.
Patients with malignant inoperable esophagus tumors of epithelial origin
Advanced esophagus tumors, essentially those of the upper one third of the esophagus have histological adenosquamous similarities with the malignant head and neck tumors, and also overexpress the EGFR. The normal treatment for advanced and non resectable tumors is the CRT, based on cisplatin with fluoruracyl. With this therapeutic regime, the rate of objective response is of 30 to 60 %, and the survival median is of less than one year [47]. In a controlled and randomized clinical trial the treatment with nimotuzumab used to counteract the EGFR in patients with esophagus malignant tumors, was higher when combined with CRT [20]; the control rate of the disease was significantly increased in 60.9 % compared to CRT alone (26.9 %). The survival median was higher in the MAb group compared to the control group: 8.1 months vs 3 months [20].
The preliminary analysis of a second controlled and random study in progress in Brazil has verified the antitumor effect of the nimotuzumab combined with CRT in patients with advanced esophagus cancer (Figure 8) [48].
Table 2 summarizes the main results of its clinical efficacy for the conditions for which it was registered.
CLINICAL EVALUATION IN OTHER ONCOLOGICAL INDICATIONS
Due to the therapeutic potential of Nimotuzumab in malignant tumors of epithelial origin, we are evaluating it in other oncological conditions, such as advanced non-small cell lung tumors, gastric tumors, colorectal tumors, liver carcinoma, pancreas carcinoma, breast cancer, cervix and prostate cancers and malignant meningiomas [50-52].
TOXICOLOGY
Immunogenicity studies
A high monkey (Cercopithecus aethiops) anti-mouse antibody response (MAMA), was obtained with the murine antibody ior egf/r3. However, with the humanized version (nimotuzumab), the response was not measurable after two immunizations, and low titers were obtained after four subcutaneous administrations of the antibody coupled to the adjuvant. This experiment demonstrated the low immunogenicity of the humanized version [53, 54].
Single dose toxicity studies
Increasing dosages of Nimotuzumab of up to 10 times the equivalent dose established for humans, 400 mg (5.71 mg/kg), were used to evaluate the toxicity of a single dose in Sprague Dawley rats. No significant signs of toxicity were observed, which could be attributed to the administration of any of the doses of the product, not even when assessing the highest dose [7].
Pharmacotoxicology study at repeated doses
Repeated daily dosages of Nimotuzumab were supplied by the intravenous route to green monkeys (Cercopithecus aethiops sabaeus) for 14 days in proportions of 2.85 or 11.4 mg/kg of body weight. This corresponded approximately to one and four times the effective dose at 50 % (ED50) for human beings: 200 mg (total dose). This study demonstrated that the administration of up to 11.4 mg/kg of nimotuzumab did not produce alterations that could be interpreted as signs of toxicity or undesirable pharmacological side effects [54].
Local tolerance
An experiment in rabbits was also carried out to assess local tolerance, in which nimotuzumab was administered by the intravenous route while using a control group. A microscopic erythema was observed near the injection site in two animals; one of them had been treated with nimotuzumab and the other belonged to the control group. Inflammation was not observed after the administration of the product in any animal. The histopathological studies did not show any intravenous irritation produced by the administration of the medication [43].
In skin biopsies of patients submitted to the intravenous administration of 200 mg of nimotuzumab, the perivascular lymphocytic inflammatory infiltrate, which is detected when using other EGFR inhibitors [17], was confirmed to be lacking in the superficial dermis.
SAFETY PROFILE OF NIMOTUZUMAB IN HUMANS
Adverse reactions in clinical trials
The adverse reactions in clinical trials before registration, most of which were shivering, nausea, headache, vomiting, anemia, arterial hypotension or hypertension, fever, increased liver enzymes (alkaline phosphatase and oxalacetic and glutamic-pyruvic transaminases).
Other less frequent adverse reactions were sleepiness, disorientation, myalgia, motor dysphasia, incoherent language, buccal dryness, face reddening, weakening of lower limbs, phlebitis, increase of creatinine, leukopenia, hematuria, thoracic pain and peribuccal cyanosis [55]. Because of their intensity, these reactions are classified as mild or moderate, according to the common toxicity criteria (CTCAE, version 3) and they disappeared with conventional treatments.
It should be stressed that nimotuzumab has been used in vulnerable populations such as the elderly and children, with good tolerance and an identical safety profile to that of the rest of the population [27, 37, 45]. This biopharmaceutical can be administered as a prolonged maintenance treatment, without any evidence of cumulative toxicity or the exacerbation of toxicity by other concomitant therapies [27, 37, 45].
Adverse criteria described in the post-marketing experience
After registering the nimotuzumab, an observational, prospective, multicenter and open clinical trial was carried out with the treatment of 577 patients with advanced tumors of epithelial origin; 89 of them were of pediatric ages and 488 adults. In 19.1 and 22.5 %, of them respectively, there was at least one adverse event observed during the treatment. The association between the exposure time to the monoclonal antibody, the number of doses applied and the frequency of the presence of adverse events was not proven. There was no influence in the intensity of the events either, regardless the causal relationship. Most of the events were classified as mild or moderate. Very few severe adverse events were notified, i.e., one patient with anaphylaxis, one with venous thrombosis, one with lipothymia and another one with tumoral lysis syndrome [37].
The overall report on nimotuzumab safety (issued in 2012) shows that out of a total of 38 629 patients, 10 % of them were treated in clinical trials and the other 90 % were treated following the doctor's prescription. There were 36 severe adverse events related to nimotuzumab; 16.7 % of them showed a definitive causal effect, 25 % were probable and 58.3 % were possible (Sierra P, 2012, personal communication). Those included within the adverse events having an incidence of 5 % or more were: vomiting (16.7 %), nausea (11.1 %), gastrointestinal hemorrhage (5.6 %); or an allergic/immunological cause such as infusion reaction (8.3 %) and anaphylaxis (5.6 %).
Table 2 and table 3 summarize the most frequent adverse events reported in clinical trials before and after the registration of nimotuzumab.
This demonstrates that the product is well tolerated as a single treatment or combined with the conventional oncological therapies, without any exacerbation of toxicity. Furthermore, it is distinguished by its infrequent dermatological toxicity, compared to other anti-EGFR [56]. This differential toxicity profile can be explained, above all, because nimotuzumab has an intermediate affinity for EGFR, which favors the binding of the MAb with cells having a higher density of receptors, such as malignant cells, compared to the normal cells with less density. Therefore, its affinity is optimized for the tumors, precisely where it achieves a bivalent binding that is an effector of the blocking of the signaling cascade for cellular proliferation, and it does not produce damage to normal epithelial cells [17, 57].
REGULATORY CONDITION
Since 2004, The European Medicine Agency (EMA) and the Food and Drug Administration (FDA) of the United States classified nimotuzumab as an "orphan drug" for pediatric gliomas. And since 2008, it is so for pancreas cancer, as defined by EMA [17]. This medication has a sanitary registration in 31 countries (until March of 2013) (Figure 9).
CONCLUSIONS
Nimotuzumab is a novel humanized MAb with an antitumor effect that is noted by the increase of the survival of patients with advanced head and neck tumors, malignant glial tumors and advanced esophagus tumors, demonstrated by controlled studies and in medical practice. Its safety profile surpasses that of other monoclonal antibodies anti-EGFR, thereby favoring its combined use with other conventional therapies, its utilization as a prolonged maintenance medication, and in vulnerable populations as of the elderly and children.
Its therapeutic potential in tumors of epithelial origin ensures the continuity of the ongoing studies on its safety and efficacy in several diseases.
REFERENCES
1. Cohen S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962;237:1555-62.
2. Cowley GP, Smith JA, Gusterson BA. Increased EGF receptors on human squamous carcinoma cell lines. Br J Cancer. 1986;53(2):223-9.
3. Ciardiello F, Tortora G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur J Cancer. 2003;39(10):1348-54.
4. Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19(56):6550-65.
5. Cohen EE. Role of epidermal growth factor receptor pathway-targeted therapy in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24(17):2659-65.
6. Agulnik M. New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN). Med Oncol. 2012;29(4):2481-91.
7. Mateo C, Moreno E, Amour K, Lombardero J, Harris W, Perez R. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology. 1997;3(1):71-81.
8. Prieto Y, Rojas L, Hinojosa L, Gonzalez I, Aguiar D, de la Luz K, et al. Towards the molecular characterization of the stable producer phenotype of recombinant antibody-producing NS0 myeloma cells. Cytotechnology. 2011;63(4):351-62.
9. Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer. 2002;101(6):567-75.
10. Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol. 2004;22(9):1646-54.
11. Crombet T, Pérez R, Lage A, Osorio M, Cruz T. Anticuerpo monoclonal humanizado h-R3: un nuevo concepto terapéutico para el tratamiento del cáncer avanzado. Biotecnol Apl. 2003;20(1):33-51.
12. Diaz Miqueli A, Blanco R, Garcia B, Badia T, Batista AE, Alonso R, et al. Biological activity in vitro of anti-epidermal growth factor receptor monoclonal antibodies with different affinities. Hybridoma. 2007;26(6):423-31.
13. Diaz Miqueli A, Rolff J, Lemm M, Fichtner I, Perez R, Montero E. Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies. Br J Cancer. 2009;100(6):950-8.
14. Akashi Y, Okamoto I, Iwasa T, Yoshida T, Suzuki M, Hatashita E, et al. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status. Br J Cancer. 2008;98(4):749-55.
15. Basavaraj C, Sierra P, Shivu J, Melarkode R, Montero E, Nair P. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther. 2010;10(7):673-81.
16. Rodriguez MO, Rivero TC, del Castillo Bahi R, Muchuli CR, Bilbao MA, Vinageras EN, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. 2010;9(5):343-9.
17. Rojo F, Gracias E, Villena N, Cruz T, Corominas JM, Corradino I, et al. Pharmacodynamic trial of nimotuzumab in unresectable squamous cell carcinoma of the head and neck: a SENDO Foundation study. Clin Cancer Res. 2010;16(8):2474-82.
18. Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. mAbs. 2009;1(1):41-8.
19. Hong J, Peng Y, Liao Y, Jiang W, Wei R, Huo L, et al. Nimotuzumab prolongs survival in patients with malignant gliomas: A phase I/II clinical study of concomitant radiochemotherapy with or without nimotuzumab. Exp Ther Med. 2012;4(1):151-7.
20. Ramos-Suzarte M, Lorenzo-Luaces P, Lazo NG, Perez ML, Soriano JL, Gonzalez CE, et al. Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther. 2012;13(8):600-5.
21. Solomon MT, Selva JC, Figueredo J, Vaquer J, Toledo C, Quintanal N, et al. Radiotherapy plus the anti-EGFR mAb nimotuzumab or placebo for the treatment of high-grade glioma patients [Abstract]. J Clin Oncol. 2012;30(suppl):2515.
22. Zhao XY, Guo Y, Zhu YX, Wang Y, Zhu GP, Hu CS, et al. Clinical analysis of nimotuzumab plus cisplatin and fluorouracil regimen as induction treatment in resectable head and neck squamous cell carcinoma. Chinese J Otorhinolaryngol Head Neck Surg. 2012;47(7):536-9.
23. Crombet T, Torres L, Neninger E, Catala M, Solano ME, Perera A, et al. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother. 2003;26(2):139-48.
24. Bode U, Massimino M, Bach F, Zimmermann M, Khuhlaeva E, Westphal M, et al. Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther. 2012;12(12):1649-59.
25. Centro para el Control Estatal de Medicamentos, Equipos y Dispositivos Médicos. Registro sanitario de medicamentos. La Habana: Cecmed. c2012[cited 2013 Dec 4]. Available from: http://www.cecmed.cu/Pages/RegSan.htm.
26. Meng J, Gu QP, Meng QF, Zhang J, Li ZP, Si YM, et al. Efficacy of nimotuzumab combined with docetaxel-cisplatin-fluorouracil regimen in treatment of advanced oral carcinoma. Cell Biochem Biophys. 2014;68(1):181-4.
27. Cabanas R, Saurez G, Rios M, Alert J, Reyes A, Valdes J, et al. Treatment of children with high grade glioma with nimotuzumab: a 5-year institutional experience. mAbs. 2013;5(2):202-7.
28. Yan S, Jiang X, Yang J, Yan D, Wang YX. Radiotherapy for nasopharyngeal carcinoma and combined capecitabine and nimotuzumab treatment for lung metastases in a liver transplantation recipient: a case experience of sustained complete response. Cancer Biother Radiopharm. 2012;27(8):519-23.
29. Qi DL, Wang HQ, Li Y, Huang CB, Wang QS, Xu L, et al. [fficacy and adverse effets of nimotuzumab plus palitaxel liposome and carboplatin in the treatment for advanced non-small cell lung cancer. Chinese J Oncol. 2012;34(2):152-5.
30. Li LF, Wang HQ, Liu XM, Zhang HL, Qiu LH, Qian ZZ, et al. Nimotuzumab in combination with chemotherapy in patients with advanced non-small cell lung cancer. Chinese J Oncol. 2011;33(8):626-8.
31. Ling Y, Chen J, Tao M, Chu X, Zhang X. A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis. 2012;4(1):58-62.
32. Verduzco-Rodriguez L, Aguirre-Gonzalez EH, Verduzco-Aguirre HC. Durable complete response induced by paclitaxel-nimotuzumab-methotrexate chemotherapy in a patient with metastatic head and neck squamous cell carcinoma. Hematol Oncol Stem Cell Ther. 2011;4(4):182-4.
33. Zhao KL, Hu XC, Wu XH, Fu XL, Fan M, Jiang GL. A phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagus. Invest New Drugs. 2012;30(4):1585-90.
34. Winquist E, Nabid A, Sicheri D, Ganguly P, Venkatesan V, Schneider K, et al. A phase I dose escalation study of a humanized monoclonal antibody to EGFR (hR3) in patients with locally advanced squamous cell cancer of the head and neck (SCCHN) treated with radiotherapy (RT) [Abstract]. Proc Am Soc Clin Oncol. 2002;21:91a.
35. Huang XD, Yi JL, Gao L, Xu GZ, Jin J, Yang WZ, et al. Multi-center phase II clinical trial of humanized anti-epidermal factor receptor monoclonal antibody h-R3 combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Chinese J Oncol. 2007;29(3):197-201.
36. Babu K, Joseph B, Vidyasagar MS, Bonanthaya R, Pasha CT, Bapsy PP et al: An open-label, randomized, study of h-R3mAb (nimotuzumab) in patients with advanced (stage III or IVa) squamous cell carcinoma of head and neck (SCCHN): Four-year survival results from a phase IIb study [Abstract]. J Clin Oncol 2010;28(15 Suppl):428s.
37. Piedra P, Morejón O. Report of the Fifth Nimotuzumab Global Meeting. Biotecnol Apl. 2010;27(1):56-61.
38. Piedra P, Saurez G, Barroso M, Ledón N. Observational clinical study in patients with advanced stage epithelial tumors treated with nimotuzumab [Abstract]. Can J Clin Pharmacol. 2010;17(1):e234.
39. Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) Classification of Tumours of the Central Nervous System: newly codified entities. Brain Pathol. 2007;17(3):304-7.
40. Lorenzoni J, Torrico A, Villanueva P, Gederlini A, Torrealba G. Surgery for high-grade gliomas in a developing country: survival estimation using a simple stratification system. Surg Neurol. 2008;70(6):591-7; discussion 7.
41. Ramos TC, Figueredo J, Catala M, Gonzalez S, Selva JC, Cruz TM, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase I/II trial. Cancer Biol Ther. 2006;5(4):375-9.
42. Westphal M, Bach F. Final results of a randomized phase III trial of nimotuzumab for the treatment of newly diagnosed glioblastoma in addition to standard radiation and chemotherapy with temozolomide versus standard radiation and temoziolamide. J Clin Oncol. 2012;30(15 suppl 1):2033.
43. Centro de Inmunología Molecular. Estudio de toxicidad local de nimotuzumab en conejos. La Habana: Centro de Investigación de Evaluaciones Biológicas (CIEB); 1997.
44. Packer RJ. Progress and challenges in childhood brain tumors. J Neurooncol. 2005;75(3):239-42.
45. Saurez G, Cabanas R, Zaldivar M, Garnier T, Iglesias B, Piedra P, et al. Clinical experience with nimotuzumab in cuban pediatric patients with brain tumors, 2005 to 2007. MEDICC Rev. 2009;11(3):27-33.
46. Massimino M, Bode U, Biassoni V, Fleischhack G. Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther. 2011;11(2):247-56.
47. Wong RK, Malthaner RA, Zuraw L, Rumble RB, Cancer Care Ontario Practice Guidelines Initiative Gastrointestinal Cancer Disease Site G. Combined modality radiotherapy and chemotherapy in nonsurgical management of localized carcinoma of the esophagus: a practice guideline. Int J Radiat Oncol Biol Phys. 2003;55(4):930-42.
48. Castro G, Segalla J, Stümoller C, Schmerling R, Andrade CJ, Pinczowski H, et al. Complete response (CR) to nimotuzumab plus chemoradiation in locally advanced esophageal cancer: Preliminary results of an ongoing randomized trial. Ann Oncol. 2010;21 Suppl. 8: viii260.
49. Saurez G. Reporte del Séptimo encuentro científico internacional: Nimo-meeting 2012 en la sesión de tumores cerebrales. Rev Cubana Neurol Neurocir. 2012;2(2):171-6.
50. Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biologics. 2010;4:289-98.
51. Kim SH, Shim HS, Cho J, Jeong JH, Kim SM, Hong YK, et al. A phase I trial of gefitinib and nimotuzumab in patients with advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2013;79(3):270-5.
52. World Health Organization. International Clinical Trials Registry Platform (ICTRP). Geneva: WHO. c2013[cited 2013 Dec 4]. Available from: http://apps.who.int/ictrp/search/en/.
53. Arteaga ME, Ledon N, Casaco A, Pardo B, Garcia M, Boleda M, et al. Systemic and skin toxicity in Cercopithecus aethiops sabaeus monkeys treated during 26 weeks with a high intravenous dose of the anti- epidermal growth factor receptor monoclonal antibody Nimotuzumab. Cancer Biol Ther. 2007;6(9):1390-5.
54. Arteaga-Perez ME, Maceira M, Casaco A, Hernandez-Sosa O, Bada-Barro AM, Leon-Goni A, et al. Multiple dose toxicity study of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 intravenously administered to Cercopithecus aethiops sabaeus monkeys. Hum Exp Toxicol. 2004;23(5):219-27.
55. Cimaher (nimotuzumab). Resumen de las características del producto. Habana: CIMAB S. A. c2013 [cited 2013 Dec 4]. Available from: http://www.cecmed.cu/Pages/RCP_Med.htm.
56. Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9(9):1199-206.
57. Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther. 2011;11(4):373-82.
Received in November, 2013.
Accepted in May, 2014.
Giselle Saurez-Martínez. Departamento de Gerencia Médica y Asuntos Regulatorios, Centro de Inmunología Molecular, CIM. Calle 206 No. 1926 e/ 19 y 21, Atabey, Playa, CP 11600, La Habana, Cuba. E-mail: giselle@cim.sld.cu.