My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.31 no.3 La Habana July.-Sept. 2014
REPORT
Development of soluble and immobilized biocatalysts based on a recombinant thermostable β-fructosidase enabling complete sucrose inversion at pasteurization temperatures
Desarrollo de biocatalizadores solubles e inmovilizados basados en una β-fructosidasa recombinante termoestable que permite la inversión total de la sacarosa a temperaturas de pasteurización
Carmen Menéndez1, Duniesky Martínez2, Luis E Trujillo1, Ricardo Ramírez1, Alina Sobrino2, Bessy V Cutiño-Ávila3, Liliana Basabe4, Alberto del Monte-Martínez3, Enrique R Pérez2, Lázaro Hernández1
1 Laboratorio de Interacciones Planta-Microorganismo, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
2 Laboratorio de Fermentaciones, Centro de Ingeniería Genética y Biotecnología de S. Spíritus. Circunvalante Norte S/N, Olivos 3, CP 60200, Sancti Spíritus, Cuba.
3 Laboratorio de Tecnología de Enzimas, Centro de Estudio de Proteínas, Facultad de Biología, Universidad de La Habana, UH. Calle 25 entre J e I, Vedado, La Habana, CP 10400, Cuba.
4 Laboratorio de Biotecnología Acuática, Dirección de Investigaciones Agropecuarias, CIGB, La Habana, Cuba.
ABSTRACT
Biocatalysts for the industrial production of invert sugar are preferred to stably operate at high sucrose concentrations and pasteurization temperatures. Thermotoga maritima β-fructosidase (BfrA) is more thermostable and less susceptible to substrate inhibition than the current commercial invertase from Saccharomyces cerevisiae. In this research, the non-saccharolytic host Pichia pastoris was engineered for BfrA production. Fed-batch fermentation of the recombinant yeast for 72 h using cane sugar as a non-expensive energy source yielded cultures of cell densities over 100 g/L (dry biomass) with invertase activity exceeding 300 U/mL. BfrA was secreted to the cell periplasmic space and the culture medium as a fully active glycoprotein with unaltered thermostability. The extracellularly-released BfrA representing 85 % of the total proteins in the culture supernatant was either dried into powder to generate a soluble free enzyme biocatalyst (specific activity 15 000 U per gram of powder) or covalently immobilized on Glyoxyl-Sepharose CL 4B to generate an insoluble enzyme biocatalyst (specific activity 9249 U per gram of dry support) for reuse. As a third approach, the biomass bearing the periplasmic BfrA was submitted to a killing heat treatment and entrapped in calcium alginate beads to generate a reusable non-viable cell biocatalyst (specific activity 103 U per gram of dry beads). The three biocatalysts completely hydrolyzed cane sugar (70 %, w/v) in batchwise or continuous operation at 60 ºC, offering alternative cost-effective options for the industrial manufacture of food-grade inverted sugar syrup. This research granted the 2013 Award of the Cuban National Academy of Sciences.
Keywords: invertase, invert syrup, immobilization, Thermotoga maritima, Pichia pastoris.
RESUMEN
El empleo de biocatalizadores termoestables en la producción industrial de sirope invertido es aún una meta por lograr. La enzima b-fructosidasa (BfrA) de Thermotoga maritima es más termoestable y menos susceptible a la inhibición por sustrato que la invertasa comercial de Saccharomyces cerevisiae. En esta investigación, Pichia pastoris se modificó genéticamente para producir BfrA. La levadura recombinante alcanzó densidad celular superior a 100 g/L (biomasa seca) y actividad invertasa por encima de 300 U/mL en fermentaciones de 72 h con el empleo de sacarosa como fuente barata de carbono. BfrA se secretó al periplasma celular y al medio de cultivo en forma de una glicoproteína totalmente activa y termoestable. BfrA con pureza de 85 % en el sobrenadante de cultivo se secó hasta polvo para generar un biocatalizador de enzima soluble (actividad específica 15 000 U/g peso seco) o se inmovilizó de forma covalente al soporte Glioxil-Sepharosa CL 4B para generar un biocatalizador insoluble (actividad específica 9249 U/g peso seco) que permita reuso. En un tercer enfoque, la biomasa celular fue sometida a un tratamiento térmico y encapsulada en esferas de alginato de calcio para generar un biocatalizador reusable de células muertas (actividad específica 103 U/g peso seco). Los tres biocatalizadores hidrolizaron completamente el azúcar de caña (70 % p/v) en operación continua o discontinua a 60 ºC, y ofrecen opciones alternativas rentables para la inversión enzimática del azúcar a escala industrial. Este trabajo mereció el Premio Anual de la Academia de Ciencias de Cuba para el año 2013.
Palabras clave: invertasa, sirope invertido, inmovilización, Thermotoga maritima, Pichia pastoris.
INTRODUCTION
An ideal biocatalyst for the enzymatic manufacture of invert sugar should optimally operate in a highly concentrated sucrose solution (70 %, w/v) to minimize the initial dilution and final concentration steps and at pasteurization temperatures (60-70 °C) to improve substrate diffusion and minimize microbial contamination. Saccharomyces cerevisiae has been for years the source of commercial invertase. The enzyme is secreted to the yeast periplasm but suffers from substrate inhibition at sucrose levels above 20 % (w/v) and its operational stability in soluble or immobilized biocatalysts is rather low at temperatures exceeding 50-55 °C [1, 2].
Thermotoga maritima β-fructosidase (BfrA) is the most thermoactive and thermostable invertase so far identified and it is less susceptible to substrate inhibition than the S. cerevisiae enzyme. Due to these properties, BfrA constitutes an attractive candidate for recombinant production and use in the manufacture of invert sugar [3-5].
Immobilized biocatalysts offer advantages over soluble biocatalysts in terms of enzyme reuse, easier separation of the enzyme from the products, and more flexible reactor configurations. Glyoxyl-agarose beads have been successfully employed for the covalent immobilization of different enzymes, resulting in high stabilization factors and high recovery of enzyme activity after immobilization [6]. Entrapment in insoluble calcium alginate gel is recognized as a simple, inexpensive and non-toxic method for immobilization of cells and enzymes with applications in the food and pharmaceutical industries [7]. The entrapment of whole cells bearing periplasmic invertase activity prevents the enzyme from leaking out of the alginate beads, but may cause operational troubles during sucrose inversion if the confined cells remain alive. The release of the fermentation by-products ethanol and acetate affects the syrup quality while the formation of CO2 bubbles increases the internal pressure of packed-bed columns which then tend to crack [8].
The yeast Pichia pastoris has been widely used for heterologous protein production [9]. This non-saccharolytic host with the GRAS status is particularly appropriate for the secretion of sucrose-modifying enzymes with applications in the food and pharmaceutical industries [10-12]. In this study, P. pastoris was engineered to secrete high levels of N-glycosylated BfrA. The extracellularly released enzyme and the whole cells with periplasmic BfrA activity were used to develop three types of thermostable biocatalysts that completely hydrolyzed cane sugar (70 %, w/v) in batchwise and continuous operation at pasteurization temperatures. Our results have both academic and applied relevance in the field of industrial biotechnology. This research granted the 2013 Award of the Cuban National Academy of Sciences.
RESULTS AND DISCUSSION
Production of N-glycosylated T. maritima β-fructosidase (BfrA) in recombinant P. pastoris by fed-batch fermentation
A synthetic bfrA gene with codon usage modified for optimal host translation was fused to the alpha factor signal peptide of vector pGAPZaC (Invitrogen) and expressed in Pichia pastoris under the control of the constitutive GAP promoter and the AOX1 terminator. The gradual increase of the transgene dosage from one to four copies of the expression cassette had an additive effect on BfrA yield without causing cell toxicity. The bfrA expression levels in the multicopy Pichia clones remained stable for at least 100 generations during continuous growth in the absence of the selection antibiotic zeocin. The four-copy strain PpBfrA(4×) showed high invertase activity in the cell periplasmic space (approximately 2000 U/g of dry undisrupted biomass) and the culture supernatant (approximately 150 U/mL) with a global volumetric productivity around 5700 U/L/h in fed-batch fermentation using cane sugar as the sole carbon source. The engineered yeast grew on sucrose by a respiratory route achieving average yield coefficient of 0.60 g dry biomass per gram of sucrose consumed. The average cell density after fermentation for 72 h exceeded 100 g/L (dry biomass).
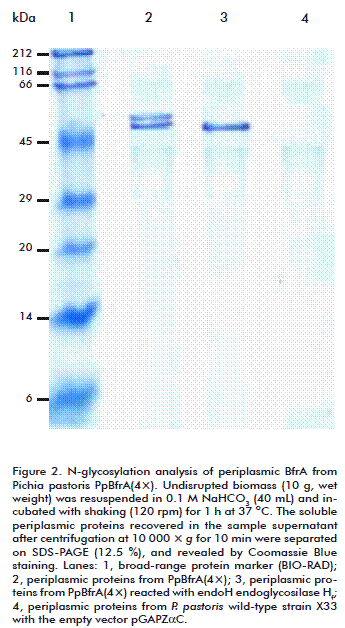
BfrA purified either from the cell periplasm or the culture supernatant migrated as two bands on SDS-PAGE with estimated molecular masses of 53 and 58 kDa (Figure 1 and Figure 2). A treatment with endoglycosidase Hf produced a single protein band with the expected size of 51 kDa. This result indicates that BfrA is secreted to the periplasmic space as a glycoprotein with two different glycosylation degrees and then it is released to the external medium without further modification. The presence of N-linked oligosaccharides did not alter the optimal activity, thermal stability, substrate specificity, and kinetic properties of recombinant BfrA, which hydrolyzed sucrose with a catalytic efficiency (kcat/KM) of 5.6 × 104 M-1 s-1 at pH 5.5 and 75 ºC. This value is similar to that reported for the unglycosylated enzyme produced in Escherichia coli [3, 4].
Development of a soluble BfrA biocatalyst and batch hydrolysis of cane sugar
The fully active N-glycosylated BfrA represented 85 % of the total amount of proteins released to the external medium by PpBfrA(4×) at the end of the fed-batch fermentation. Free BfrA was first used to develop a thermostable soluble biocatalyst following the steps described below. The yeast cells remaining in the culture supernatant after centrifugation were removed in two consecutive microfiltration steps using filters with pore size of 0.45 and 0.2 mm, respectively. The cell-free filtrate was concentrated with continuous dialysis against 50 mM sodium acetate buffer (pH 5.5) by ultrafiltration using a 30 kDa cut-off membrane and submitted to a drying rotoevaporation process at 50-60 ºC. The resulting BfrA powder with average specific invertase activity of 15 000 U/g and protein purity above 90 % was fully stable at the assayed storage temperatures of 4 and 30 ºC for at least 10 months.
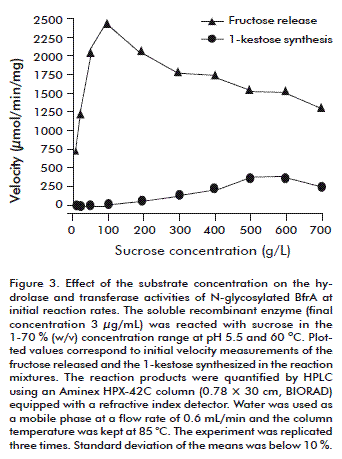
Soluble BfrA was reacted with sucrose in the 1-70 % (w/v) concentration range at pH 5.5 and 60 ºC in a 1-L batch reactor with stirring at 250 rpm (Figure 3). Initial velocity measurements show that hydrolysis occurred gradually faster as the substrate concentration was driven from 1 to 10 % (w/v). The enzyme suffered from slight inhibition at sucrose levels above 20 % (w/v). Under these conditions, the velocity of sucrose hydrolysis decreased and 1-kestose [αglu(1,2)βfru(1,2)bfru)] was synthesized as a result of the transfructosylating side reaction of the enzyme. At the highest assayed substrate concentration (70 %, w/v), the overall BfrA activity (hydrolase plus transferase) was 1.6-fold reduced and 1-kestose represented 19 % of the total products at initial reaction rates. As the reactions proceeded, sucrose and the synthesized trisaccharide were completely hydrolyzed (data not shown).
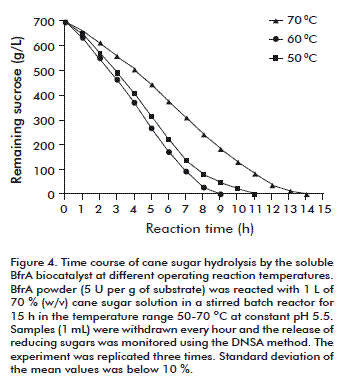
Time-course analyses of batch sucrose hydrolysis by the soluble BfrA biocatalyst were then conducted in the temperature range of 50-70 ºC using an enzyme dose of 5 U/g of cane sugar at an initial concentration of 70 % (w/v) and constant pH 5.5 (Figure 4). Due to its high specific activity, the BfrA powder added negligible mass (4.2 g; 3500 U) to the reaction volume (1 L). The complete substrate inversion took 9, 11 and 14 h for the reactions at 70, 60 and 50 ºC, respectively. The average productivity in the reaction at 70 ºC was 15.8 g of substrate hydrolysed/enzyme unit/hour.
The highest productivity value of 20.3 was reached at the reaction time interval between 4 and 5 h when half of the initial substrate content had been transformed. The incubation at 70 ºC promoted the occurrence of Maillard browning reactions, while the products solution remained colourless in the experiments at 60 and 50 ºC.
The thermostable soluble BfrA biocatalyst can have a prolonged reuse in batch reactions at 60 ºC if it is operated in a membrane bioreactor. For this purpose, the tank output port is recommended to be coupled to a cartridge-type ultrafiltration membrane with 30-kDa cutoff to retain the enzyme inside the reactor after discharging the product solution.
Rational immobilization of BfrA on Glyoxyl-Sepharose CL 4B
Recombinant BfrA containing the C-terminal polyhistidine tag was purified by Ni affinity chromatography from the culture supernatant and optimally bound to Glyoxyl-Sepharose CL 4B using the Rational Design of Immobilized Derivatives (RDID) strategy. Multipoint-covalent attachment of the N-glycosylated BfrA onto the activated support at pH 10 allowed total recovery of the loaded enzyme and its activity. The immobilization process caused no variation in the catalytic properties of the enzyme and further enhanced the thermal stability. The immobilized enzyme biocatalyst showed average specific activity around 9249 U/g (dry weight).
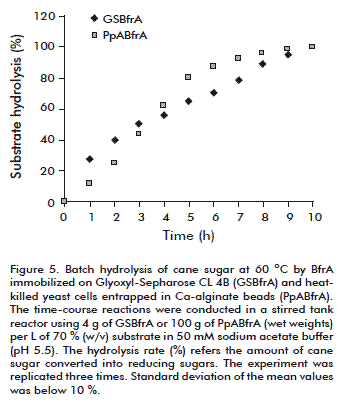
Complete inversion of cane sugar (70 %, w/v) in a batch stirred tank reactor at 60 °C was achieved with a productivity of 22.2 g of substrate hydrolysed/g of biocatalyst/h (Figure 5) [13].
Calcium alginate entrapment of heat-killed cells with periplasmic BfrA activity
P. pastoris PpBfrA(4×) cells bearing periplasmic BfrA activity were submitted to a heat-killing treatment (30 min at 70 ºC) prior to calcium alginate entrapment to prevent the occurrence of sucrose fermentation, an undesirable process which causes substrate loss and the formation of the by-products ethanol and carbon dioxide. The invertase activity of the non-viable cells (3092 U/g, dry weight) increased almost 1.2-fold in comparison to the untreated biomass (2603 U/g, dry weight) due to an improved diffusion of the substrate and products through the yeast cell wall. Maximal values of immobilization yield (99.6 %) and specific invertase activity (103 U/g of dry beads) were achieved with biomass loading of 300 g/L (wet weight). The immobilization of more concentrated biomass (400 g/L) restricted the internal diffusion of the substrate sucrose resulting in calcium-alginate beads of lower specific activity (90.7 U/g dry beads).
Batch hydrolysis of cane sugar (70 %, w/v) by the entrapped cells was conducted in a laboratory-scale stirred reactor operating at 60 ºC. The complete sucrose inversion took 10 h with average productivity of 4.37 g of substrate hydrolysed/g of dry beads/h (Figure 5). The beads retained 47 % of its original invertase activity after recycling in a stirred tank reactor for 15 days. The half-life of BfrA in the entrapped cells during batchwise operation at 60 ºC was 14 days [14].
Thermal stability of the immobilized biocatalysts during continuous production of invert sugar
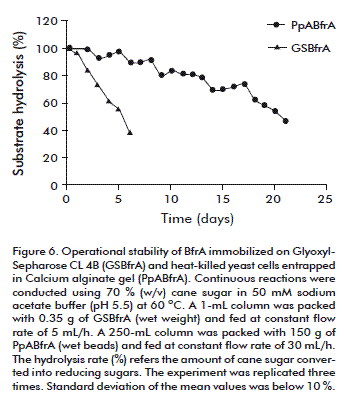
The operational stability of BfrA immobilized on Glyoxyl-Sepharose CL 4B and the heat-killed yeast cells entrapped in calcium alginate gel was evaluated for continuous hydrolysis of sugar cane (70 %, w/v) at pH 5.5 and 60 ºC (Figure 6). The conversion efficiency of the covalently immobilized enzyme dropped to 96.7 % and 45.2 % after operation with constant feeding flow of 5 mL/h for 1 and 6 days, respectively. The entrapped cells were fed at a higher constant flow rate (30 mL/h) and retained 48 % of the initial BfrA activity after 21 days of reuse. The half-life values of the invertase activity of the immobilized enzyme and the entrapped cells with continuous reuse in the fixed-bed reactors at 60 °C were estimated to be 5 and 20 days, respectively [13, 14].
We do not exclude the possibility that the support with the covalently bound enzyme may have partially escaped through the column filter during operation.
RELEVANCE OF THE STUDY
An ideal immobilized biocatalyst for the industrial scale production of invert sugar should allow the complete hydrolysis of the substrate sucrose at high concentrations with stable operation under pasteurization conditions. Current soluble or immobilized biocatalysts based on the thermolabile invertase from Saccharomyces cerevisiae are operated at temperatures not exceeding 50 ºC and exhibit substrate inhibition kinetics. T. maritima β-fructosidase (BfrA) is the most thermoactive and thermostable sucrose-hydrolysing enzyme so far identified and it is less sensible to substrate inhibition than the commercial yeast invertase.
In this research, we have developed three thermostable biocatalysts using BfrA produced as a fully active glycoprotein in a recombinant P. pastoris strain. The biocatalysts consisting of a soluble enzyme powder, a covalently immobilized enzyme, or heat-killed yeast cells entrapped in Ca-alginate beads fully hydrolyzed cane sugar (70 %, w/v) to an equimolar mixture of glucose and fructose in a resulting solution which was free of by-products and microbial contamination. The highly concentrated and colorless invert syrup does not require downstream processing prior to use in the food industry. The soluble BfrA powder adds negligible volume to the stirred batch reactor and allows short-time reactions due to its high specific activity and the absence of diffusional barriers between the substrate and the free enzyme. The immobilized enzyme is most appropriate for continuous sucrose hydrolysis in a fixed-bed reactor, while the entrapped non-viable cells can be extensively reused either in batchwise or continuous operation at 60 ºC. The three thermostable biocatalysts developed in this research offer alternative operational options for the cost-effective manufacture of food-grade inverted sugar syrups at industrial scale.
REFERENCES
1. Kotwal SM, Shankar V. Immobilized invertase. Biotechnol Adv. 2009;27(4):311-22.
2. Andjelković U, Pićurić S, Vujčić Z. Purification and characterisation of Saccharomyces cerevisiae external invertase isoforms. Food Chem.2010;120(3):799-804.
3. Liebl W, Brem D, Gotschlich A. Analysis of the gene for beta-fructosidase (invertase, inulinase) of the hyperthermophilic bacterium Thermotoga maritima, and characterisation of the enzyme expressed in Escherichia coli. Appl Microbiol Biotechnol. 1998;50(1):55-64.
4. Muñoz-Gutiérrez I, Rodríguez-Alegría ME, López-Mungía A. Kinetic behavior and specificity of b-fructosidases in the hydrolysis of plant and microbial fructans. Proc Biochem. 2009;44(8):891-8.
5. Menendez C, Martinez D, Trujillo LE, Mazola Y, Gonzalez E, Perez ER, et al. Constitutive high-level expression of a codon-optimized beta-fructosidase gene from the hyperthermophile Thermotoga maritima in Pichia pastoris. Appl Microbiol Biotechnol. 2013;97(3):1201-12.
6. Pedroche J, Yust MM, Mateo C, Fernández-Lafuente R, Girón-Calle J, Alaiz M, et al. Effect of the support and experimental conditions in the intensity of the multipoint covalent attachment of proteins on glyoxyl-agarose supports: correlation between enzyme-support linkages and thermal stability. Enzyme Microb Technol. 2007;40(5):1160-6.
7. Strand BL, Morch YA, Skjak-Braek G. Alginate as immobilization matrix for cells. Minerva Biotecnol. 2000;1(4)2:222-33.
8. Najafpour G, Younesi H, Ismail KSK. Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresour Technol. 2004;92(3):251-60.
9. Potvin G, Ahmad A, Zisheng Z. Bioprocess engineering aspects of heterologous protein production in Pichia pastoris: A review. Biochem Engineering J. 2012;64:91-105.
10. Menéndez C, Hernández L, Banguela A, País J. Functional production and secretion of the Gluconacetobacter diazotrophicus fructose-releasing exo-levanase (LsdB) in Pichia pastoris. Enzyme Microb Technol. 2004;34(5):446-52.
11. Trujillo LE, Arrieta JG, Dafhnis F, Garcia J, Valdes J, Tambara Y, et al. Fructo-oligosaccharides production by the Gluconacetobacter diazotrophicus levansucrase expressed in the methylotrophic yeast Pichia pastoris. Enzyme Microb Technol. 2001;28(2-3):139-44.
12. Trujillo LE, Gómez R, Banguela A, Soto M, Arrieta JG, Hernández L. Catalytical properties of N-glycosylated Gluconacetobacter diazotrophicus levansucrase produced in yeast. Electron J Biotechnol. 2004;7(2):5-23.
13. Martínez D, Cutiño-Avila B, Pérez ER, Menéndez C, Hernández L, del Monte-Martínez A. A thermostable exo-b-fructosidase immobilized through rational design. Food Chem. 2014;145(5):826-31.
14. Martinez D, Menendez C, Echemendia FM, Perez ER, Trujillo LE, Sobrino A, et al. Complete sucrose hydrolysis by heat-killed recombinant Pichia pastoris cells entrapped in calcium alginate. Microb Cell Fact. 2014;13:87.
Received July, 2014.
Accepted December, 2014
Lázaro Hernández. Laboratorio de Interacciones Planta-Microorganismo, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba E-mail: lazaro.hernandez@cigb.edu.cu.