My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.33 no.2 La Habana Apr.-June 2016
FOCUS
BluBAC system for determining microbial growth in clinical microbiological diagnosis samples by combining photostimulation and turbidimetry
Sistema BluBAC para la determinación del crecimiento microbiano en muestras para el diagnóstico clínico microbiológico mediante fotoestimulación y turbidimetría
Nardo Ramírez-Frómeta1, Carlos A Lamothe-Nuviola1, Elier Riverón-Rodríguez1, Carmen Y Moreno-Barrios1, Angel Regueiro-Gómez2, Carmelo J Felice3
1 Centro Nacional de Investigaciones Científicas, CNIC. Ave. 25 y Calle 158, Playa, Apartado Postal 6412, La Habana, Código Postal 10600, Cuba.
2 Centro de Bioingeniería, CEBIO, Instituto Superior Politécnico José Antonio Echeverría, ISPJAE, La Habana, Cuba.
3 Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina.
ABSTRACT
An experimental Workstation was designed, named BluBAC, intended for the microbiological diagnosis in clinical samples, which integrates photostimulation in two zones of the visible light spectrum: red (625 to 644 nm) and blue (430 to 480 nm), together with turbidimetric determinations in biological samples. It allows the analysis of photostimulation on microbial growth. The workstation comprises optoelectronic components and a MSP430 family microcontroller, connected through a Usb port to a computer for processing and visualizing the output signals com-ing from the samples. The signals are obtained through a graphic control interface in Visual Studio. The influence of the stimulation parameters (wavelength, light intensity, frequency and intensity of stimulation) on the growth of Escherichia coli cells was studied in bacterial cell cultures in DKD medium. The combination of photostimulation with turbidimetric determinations facilitated microbial detection, with decreased lag time and a longer exponential growth phase, stimulating bacterial growth more than other methods previously reported. These results evidenced the potential advantages of the experimental workstation BluBAC over other clinical microbiological diagnostic systems available in the market.
Keywords: photostimulation, turbidimetric method, clinical microbiological diagnosis, blue light, Escherichia coli, BluBAC.
RESUMEN
Se diseñó la estación de trabajo experimental BluBAC para el diagnóstico microbiológico en muestras clínicas, que integra la fotoestimulación en dos zonas del espectro luminoso visible: rojo (630 nm) y azul (de 430 a 480 nm) y permite la medición de turbidez en las muestras biológicas. De esta forma se puede analizar la influencia de la fotoestimulación en el crecimiento microbiano. La misma está formada por dispositivos optoelectrónicos y un microcontrolador de la familia MSP430 conectado a un ordenador a través de un puerto USB, en el que se procesan y grafican las señales de salida de las muestras, dichas señales obtenidas a través de una interfaz de control en Visual Studio. Se estudió la influencia de los parámetros del estímulo aplicado (longitud de onda, intensidad luminosa, frecuencia de estimulación e intensidad del estímulo) sobre el crecimiento de células de Escherichia coli cultivada en medio DKD. Se observó que la combinación de la fotoestimulación con la determinación de turbidez facilita la detección microbiana con un tiempo de latencia menor y una fase exponencial de mayor duración, lo que permitió estimular el crecimiento del microorganismo mucho más que mediante otros métodos previamente reportados. Estos resultados evidenciaron las ventajas potenciales de la estación experimental BluBAC sobre otros sistemas disponibles comercialmente para el diagnóstico microbiológico en muestras clínicas.
Palabras clave: fotoestimulación, método turbidimétrico, diagnóstico clínico microbiológico, luz azul, Escherichia coli, BluBAC.
INTRODUCTION
Photostimulation is the process by which light is absorbed by cellular molecules and subsequently converted into energy. When a cell is photostimulated, the molecules bearing conjugated double bonds absorb light at a particular wavelength set by its chemical composition and attained a higher energy state. Then, those energized and excited molecules transfer the excess energy to other nearby molecules, triggering a series of biochemical processes. Among the metabolic processes and biochemical reactions susceptible to photostimulation effects at different wavelengths there are the synthesis of adenosine triphosphate (ATP) and the synthesis of growth factors, among others [1].
Hence, the resulting effects in cell function from the increased synthesis of ATP, which is the main cell energetic intermediary molecule, and also the increased production of reactive oxygen species (ROS), initiate signal transduction cascades leading to the downstream expression of growth factors and ultimately to cell proliferation. Particularly, ROS affect homeostasis parameters such as cellular pH which alters cell functions, calcium ion concentrations which stimulate the different signaling pathways, including the activation of photoreceptors and also growth factors production [2]. It is known that small changes in ATP concentrations could significantly affect the cell metabolism, since an increased energetic state could improve the metabolic performance even in cells undergoing deleterious processes [3, 4].
Moreover, a series of experiments have demonstrated that ATP is not solely implied in a higher energetic state but also for an increased signaling among cells in multicellular organisms [5]. Therefore, the photostimulation of ATP is fundamental to understand the ubiquitous effects of photoaception mechanisms [5, 6].
Seven types of photoaceptor proteins have been described so far: rhodopsins; xantopsins; phytochromes; cryptochromes; LOV-domain (light, oxygen and tension domain) carrying proteins; BLUF (blue light utilizing flavine adenosine dinucleotide) domain proteins and the family of eight proteins resistant to ultraviolet light [7]. Of them, blue light, that is the one of higher frequency for simulation, is perceived by cryptochromes, LOV-domain and BLUF-domain proteins, which have been characterized in detail through biochemical and biophysical methods from photon absorption downstream the signal transduction cascades to the final biological activities [8]. After the initial photoexcitation event, intrinsic structural changes are induced in photoreceptors, such as the formation of double covalent linkage of the chromophore [9].
There have been described methods to take advantage of the photostimulation processes described above for microbial manipulation with varied purposes. For instance, a method was patented to stimulate the metabolism of non-phytotrophe microorganisms with blue light [10], those microorganisms been applied in bioremediation strategies or in biotechnological process for the production of biomolecules. However, such effects on microbial growth could be also used for diagnostic application in microbial clinical samples, particularly in those pathogens susceptible to photostimulation.
Therefore, in this work is described the design of the experimental workstation BluBAC, intended for microbial diagnostics in clinical samples. The effect of photostimulation on cell growth in biological samples susceptible to it for diagnostic purposes was studied through the stimulation of microorganisms in the 430-480 nm wavelength range [11]. Moreover, procedures were established for the fast detection of bacterial growth after its photostimulation, particularly in liquid medium, characterized through optical stimulation parameters (wavelength, light intensity, stimulation frequency and intensity).
DESIGN OF THE BLUBAC EXPERIMENTAL WORKSTATION
The BluBAC experimental (Figure 1A) was designed to study the effects of photostimulation on different microbial strains. It is composed of the TURB-Z electronic control and data acquisition card, together with an interface for computer programmable control. The TURB-Z board was organized in two sections, one to generate the photostimulatory signal and the other for the reception and measure of the resulting signal.
The stimulation block generates a low level signal delivered from 5 LED lamps to the biological samples to be analyzed. Each LED stimulates a single turbidimetric measuring well in the sample platen, adjusted to provide simultaneously the same stimulatory signal (Figure 1B). The signal is controlled attending to four main parameters: wavelength, frequency, light intensity (candela) and stimulus light intensity (current). The stimulation block generate stimulatory and also reference signals, for what it includes a tension-current conversion unit (Howland’s generator) and two AD9834 circuits to generate the respective stimulatory and reference signals for the demodulation process. This device is a low power (20 mW) direct digital synthesizer (DDS) able to generate high quality sinusoidal and triangular signals [12].
The reception unit measures the intensity of the light signal passing through the sample by means of an opto-electronic integrated circuit OPT-301, which includes a photodiode and a monolithic transimpedance amplifier. The output of the photodetector is connected to a synchronous demodulation unit (AD630), which filters the signal and converts it with the aid of a lock amplifier, synchronizing the signal for the subsequent demodulation process. The processing unit (minicontroller MSP430FG4618) generates an average value from readings to minimize the errors derived from coupling interferences.
Turbidimetry was implemented based on light absorption as a function of the concentration of bacterial cells, in compliance with the Lambert-Beer’s law [13], according to the equation 1:

The acquisition channel employs an AD630 demodulator to obtain the results, by integrating the turbidimetric method and the photostimulation (Vsm) with the aid of a sinusoidal reference signal (Vref), responding to the equation:
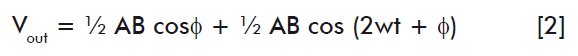
Where: A, amplitude of the imput signal; B, amplitude of the reference signal; and w, angular frequency.
A low pass filter (structure Sallen & Key, Butterwort, second order and a 2-Hz threshold frequency) eliminates the 2 wt components (Figure 1C) from the output readings, guaranteeing that the input tension of the A/D converter to depend solely on the amplitude (A), this parameter proportionally related to the absorbance of the sample (i.e., cell concentration in the biological sample). The A/D converter (resolution 10 b) acquires the data and the attached microcontroller (Figure 1D) send them to a PC through a USB port.
The workstation includes an interface in the PC (Figure 1E) to run the equipment and also to monitor in real time the growth curve of microorganisms, with three operation windows: configuration of acquisition parameters, management of the measuring system and data analysis.
ANALYSIS OF THE INFLUENCE OF OPTICAL STIMULATION PARAMETERS ON THE MEASUREMENT OF MICROBIAL SAMPLES
Experiments were run on samples of Escherichia coli strain ATCC 25923. Suspensions of bacterial cells were prepared in DKD medium at an initial concentration of 104 c.f.u./mL, as determined by colony plate counts. Cells were cultured for at 37 ºC for 18 h in a Memmert incubator model INE 700, and growth curves were established by turbidimetry readings at 5-min intervals.
The cell suspensions were continuously stimulated with low intensity blue light (1.8, 7 or 11 cd) at 470 nm, or red light in the wavelength range of 625 to 644 nm at 7, 11 or 21 cd.
As shown in figure 2A, the change of stimulation with red light from 7 to 11 cd increases the exponential phase of the growth curve and decreases the duration of the lag phase. Nevertheless, the duration of the exponential growth phase decreases by increasing the intensity from 11 to 21 cd. This indicates a sort of saturation effect in the photoreception mechanism. In general, the curves of tension over time obtained with 11 cd are statistically higher than the curves obtained at 7 and 21 cd (Figure 2A).
Otherwise, the stimulation with blue light (l = 470 nm) induces bacterial growth proportinal to light intensity. This effect not only influenced on the span of the exponential growth phase, but also reduced the duration of the lag phase, and, therefore, shortens the time required for detection. The shorter detection periods were achieved at 11 cd, as in the red light experiments (Figure 2B).
The stimulation attained at the end of the exponential phase of growth is higher with the low intensity blue light at 470 nm, as compared to that of the red light (625 nm). The results obtained at the assayed wavelengths coincide with reports for other non-photosynthetic microorganisms, in spite of using a different methodology. Greppin and Gouda re-ported a 20 to 40 % increase in the replication rate of Pseudomonas fluorescens by irradiating it with blue or red light [14, 15].
Other groups have successfully stimulated the growth of Blastocladiella emersonii [16], Candida guillermondii [17], Torula utilis [18], and Transtochytrium roseum and Sclerotinia fructigena [19], by irradiating cells with light in the range from 400 to 500 nm. Sucong et al. irradiated Corynebacterium crenatum cells with a N2 laser beam (337 nm, 6 mJ pulse energy, 10 ns and 10 Hz), and observed that cells receiving low dose radiations (< 258 J/cm2) increased cell division rates, cellular respiration, glutamic acid concentration, glutamate dehydrogenase activity and cell permeability [20]. Fedoseyeva et al. found that the activation dose following He-Ne laser irradiation was species-specific, by comparing cell growth and protein synthesis kinetics in cultures of Saccharomyces cerevisiae, Candida maltose and Candida boidinii [21, 22], and also for Torulopsis sphaerica and Endmyces magnussi [22, 23].
The abovementioned studies evidence the influence of the stimulation parameters on bacterial cell growth kinetics, further influenced by the culture conditions and the cell physiological state at the time of irradiation. In fact, experiments run by Carlyle have shown the influence of the culture medium composition on the behavior of the light irradiation, either stimulating or inhibiting the growth rate. Additionally, as found by Fedoseyeva et al. [22], oxygen consumption was determinant for the increase in cell growth after the light stimulation. Moreover, the respiratory activity of non-irradiated yeast cultures positively correlated to its susceptibility to be activated by He-Ne laser irradiation (λ = 632.8 nm), as similarly detected between the NADH-dehydrogenase activity and biomass production [24].
EFFECT OF THE FREQUENCY OF THE STIMULATION SIGNAL IN THE GROWTH RATE OF E. COLI CELLS
The frequencies in the 10 to 250 Hz range provided a higher stimulation level of cell growth rates (Figure 3A). In fact, statistically significant differences were found in the period required for detection, approximately, a 40 % reduction in the average time for detection in respect to the other analyzed frequencies. Best results were achieved at 100 Hz, this value coincidently in the activation range reported by Lloyd et al. [25], who reported that the activity of enzymes involved in physiological processes (e.g., respiration, nutrition, cell division and growth) are influenced by light frequencies in the range of 5 to 100 Hz.
INFLUENCE OF THE LIGHT INTENSITY OF THE STIMULUS ON THE E. COLI GROWTH KINETICS
The signal intensity proportionally increased the growth kinetics of E. coli cultures (Figure 3B). In fact, the increase in the current intensity not only stimulated growth significantly, but also decreased the duration of the lag phase. The 20 mA intensity was found as optimal.
THE BLUBAC SYSTEM AS AN ALTERNATIVE FOR FAST CLINICAL MICROBIAL DIAGNOSIS
The BluBAC system was developed combining the photostimulation of cells and a turbidimetric detection unit to detect cell growth. This combination is advantageous over available commercial systems for fast microbiological diagnosis, which stimulate microbial growth through biological compounds in the culture medium and control temperature and humidity changes, instead of photostimulation, and sense the effect by optical detection based on turbidity measures (Table). Such systems have two remarkable limitations: 1) the relative long periods required for detection, as compared to those obtained with the BluBAC unit (20 min; manuscript in preparation); and 2) higher costs of the equipment itself and the operation materials (30 000 to 150 000 USD; table). These negatively impacts on the economic accessibility to those systems for small or medium size laboratories, clinics and hospitals.
Additionally, the BluBAC system was validated with clinical samples from patients. In those experiments, it was found that stimulation at 470 nm similarly stimulated growth in all the microorganisms tested, what could possibly indicate that similar biological structures and processes susceptible to photostimulation could be triggered, further reducing the detection time (manuscript in preparation).
In summary, the BluBAC workstation supports high sensitivity measures of microbial growth at different wavelengths. Wavelength, light intensity, and signal frequency and intensity, as determined in E. coli, statistically influence on detection time and the microbial growth kinetics. This last parameter is modified by photostimulation, with decreased lag time, the shortage of the exponential growth phase and increased growth rate. All these results justify the good performance of the BluBAC workstation in clinical microbiology laboratories and industrial processing system.
ACKNOWLEDGEMENTS
This work was supported by the collaboration between the Bioengineering Department at the Higher Polytechnic Institute José Antonio Echeverría (IPSJAE) and the Microbiological Diagnostic Department at the National Center of Scientific Research.
REFERENCES
1. Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B.1999;49(1):1-17.
2. Kushibiki T, Tajiri T, Ninomiya Y, Awazu K. Chondrogenic mRNA expression in prechondrogenic cells after blue laser irradiation. J Photochem Photobiol B. 2010;98(3):211-5.
3. Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, et al. Mitochondrial signal introduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4(5-6):559-67.
4. Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761-71.
5. Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:332-92.
6. Khakh B, Burnstock G. The double life of ATP. Sci Am. 2009;301(6):84-92.
7. Masuda S. Light Detection and Signal Transduction in the BLUF Photoreceptors. Plant Cell Physiol. 2013;54(2):171-9.
8. Jung A, Domratcheva T, Tarutina M, Wu Q, Wen-huang Ko, Shoeman RL, et al. Structure of a bacterial BLUF photoreceptor: Insights into blue light-mediated signal transduction. Proc Natl Acad Sci USA. 2005;102(35):12350-5.
9. van der Horst MA, Hellingwerf KJ. Photoreceptor proteins, “star actors of modern times”: a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res. 2004;37(1):13-20.
10. Girinsky O, Girardin N, inventors; Girinsky O, Girardin N, assignee. Use of blue light for stimulating the metabolism of non-phototrophic microorganisms. WO2014072934A1; 2014 May 15.
11. Ramírez-Frómeta N, Lamothe-Nuviola CA, Riverón-Rodríguez E, Moreno-Barrios CY, Regueiro-Gómez A, Contreras OR. Sistema para la detección de microorganismos fotosintéticos y no fotosintéticos en muestras biológicas por fotoestimulación controlada. Patent pending, CU 2016- 0070; 2016.
12. Analog-Devices. AD9834, 20 mW Power, 2.3 V to 5.5 V, 75 MHz Complete DDS; 2014.
13. Frederic-Walton H, Reyes J. Análisis químico e instrumental moderno. Barcelona: Editorial Reverté; 1983.
14. Greppin H, Gouda S, Schorer E. Action de la lumiere sur les colonies de Pseudomonas fluorescens Mig. Arch Sci.1965;18:646.
15. Greppin H, Gouda S. Lumisynthese chez Pseudomonas fluorescens et sa nature adaptative. Mig Arch Sci.1965;18:642.
16. Cantino EC, Horenstein EA. The stimulatory effect of light upon growth and CO fixation in Blastocladiella.The SKI cycle. Mycologiya.1956;48:777-99.
17. Fraikin G, Verkhoturov V, Rubin LB. The phytochromic system in yeasts Candida guillermondii. VestnikMGU (Biol).1973;4:51-5.
18. Konev SV, Lyskova TI, Prokopova JV. Stimulative action of visible light upon division and respiration of yeast cells. Proc Ukr Acad Sci.1970;6:51-6.
19. Carlile MJ. The photobiology of fungi. Annu Rev Plant Physiol.1965;16:175-202.
20. Sucong L, Shengii Y, Dong L. Biological effects of N laser on bacterium. In: Proc. Conf. Lasers Electro-Optics Int Quant. Electron Conf. Anaheim, CA, 1990 May 21-25.
21. Fedoseyeva GE, Karu TI, Lyapunova TS, Pomoshnikova NA, Meiseel MN. Sensitivity of yeast cultures to low-intensity red light. Mikrobiologiya.1987;56:792-6.
22. Fedoseyeva GE, Karu TI, Lyapunova TS, Pomoshnikova NA, Meiseel MN. The activation of yeast metabolism with He-Ne laser radiation. I. Protein synthesis in various cultures. Lasers Life Sci.1988;2:137-46.
23. Fedoseyeva GE, Karu TI, Letokhov VS, Lobko VV, Pomoshnikova NA, Lyapunova TS, et al. Effect of He-Ne laser radiation on the reproduction rate and protein synthesis in the yeast. Laser Chem.1984;5:27-33.
24. Fedoseyeva GE, Karu TI, Lyapunova TS, Pomoshnikova NA, Meiseel MN. The activation of yeast metabolism with He-Ne laser radiation. II. Activity of enzymes of oxidative and phosphorous metabolism. Lasers LiIe Sci. 1988;2:147-54.
25. Lloyd D, Poole RK, Edwards SW. The Cell Division Cycle, Temporal Organization and Control of Cellular Growth and Reproduction. New York: Academic Press; 1982.
Received in June, 2016.
Accepted in June, 2016.
Nardo Ramírez-Frómeta. Centro Nacional de Investigaciones Científicas, CNIC. Ave. 25 y Calle 158, Playa, Apartado Postal 6412, La Habana, Código Postal 10600, Cuba. E-mail: nardo.ramirez@cnic.edu.cu.














