My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.34 no.3 La Habana July.-Sept. 2017
TECHNIQUE
Optimization of the extraction process of phenolic compounds from the brown algae Sargassum fluitans Børgesen (Børgesen)
Optimización del proceso de extracción de compuestos fenólicos del alga parda Sargassum fluitans Børgesen (Børgesen)
Richard Gutiérrez, Roberto Núñez, Lissette Quintana, Olga Valdés, Kethia González, Maria Rodríguez, Yasnay Hernández, Eudalys Ortiz
Departamento de Química, Instituto de Ciencias del Mar (ICIMAR) Calle Loma #14 e/ 35 y 37, Nuevo Vedado, Plaza de la Revolución, CP10600, La Habana, Cuba.
ABSTRACT
Sargassum fluitans is a brown seaweed species that annually arrive to Cuban shores in extensive biomass amounts. It contains considerable content of total polyphenols as structural constituents of vegetal cell walls, which can be extracted from this species for therapeutic applications as antioxidants, antiviral and anti-inflammatory agents. In previous research, there were implemented the extraction method by maceration with heat under agitation, but yields of total polyphenols were found lower than required for an efficient extraction process. Therefore, this work was aimed to optimize the extraction conditions of total polyphenol-rich compounds. For this, a complete factorial design 32 with replicates in the center of the plane was studied, together with the resulting effect of extraction time and percentage of ethanol in the extraction solution as independent variables. The response variable used was total polyphenols’ content, as determined by the Folin-Ciocalteu method. Optimal conditions were successfully established, improving yields of total polyphenols. Increaing ethanol concentration was detrimental for yields, while enlarging the extraction time was not so relevant for the efficiency of the process. The resulting regression model coefficients provided optimal design conditions, which were experimentally corroborated: 17.75 % ethanol hydroalcoholic solution (10:1 mL/g of dry seaweed) and incubation at 50 ºC under stirring at 800 rpm for 123.5 min. These conditions allowed obtaining up to 8.66 mg of total polyphenols per g of dry seaweed. These conditions are relevant to implement ef-ficient processes for polyphenols extraction from these species, as raw material for the biopharmaceutical industry..
Keywords: Sargassum fluitans, extraction, total polyphenols, optimization, factorial design.
RESUMEN
Sargassum fluitans es una alga marina parda, de la cual arriban anualmente cantidades de biomasa considerables a las costas cubanas, y fuente de polifenoles totales, que son componentes estructurales de la pared celular vegetal del alga y poseen propiedades funcionales y bioactivas como antioxidantes, antivirales y anti-inflamatorios de interés biofarmacéutico. En este trabajo se modelaron y desarrollaron las condiciones óptimas para la extracción de compuestos ricos en polifenoles totales mediante el método de maceración con agitación y calor. Para ello se aplicó un diseño factorial completo 32 con réplicas en el centro del plano y se estudió el efecto de dos factores (variables independientes) durante la extracción: el tiempo de extracción y el porcentaje de etanol en el disolvente. El contenido de polifenoles totales se determinó como variable de respuesta mediante el método de Folin-Ciocalteu. Se estableció que el incremento de las concentraciones de etanol disminuía proporcionalmente los rendimientos, sin que el tiempo de exposición de la biomasa afectara de forma notable la eficiencia del proceso. Los coeficientes de los modelos de regresión permitieron el diseño de las condiciones óptimas, las cuales fueron corroboradas experimentalmente: solución hidroalcohólica de etanol al 17.75 % (10:1 mL/g de biomasa seca) e incubación a 50 ºC durante 123.5 min con agitación a 800 rpm. Esto permitió aumentar el rendimiento de extracción de polifenoles totales hasta 8.66 mg por g de biomasa seca, lo cual permitirá implementar procesos industriales más eficientes de extracción de estos compuestos a partir de esta especie de alga.
Palabras clave: Sargassum fluitans, extracción, polifenoles totales, optimización, diseño factorial.
INTRODUCTION
Over the past twenty years, the sea has become the main natural source of bioactive molecules [1], with marine macroalgae as source of substances with novel structures and different biological activities.
Among them, brown algae produce a wide variety of secondary metabolites, which include terpenoids, oxilipins, florotanins, volatile compounds, phenolic compounds and products of mixed biogenetic origin [2, 3]. Phenolic compounds are an important group of secondary metabolites that exhibit antioxidant activity, among other biological functions. They play an important role in cellular defense of algae against abiotic and biotic stress. In fact, phenolic compounds are found in brown macroalgae such as: phenolic acids; florotanins [4] (oligomeric structures of floroglucinol (1,3,5-trihydroxybenzene) and their polymerized derivatives)[5]; polysaccharides like alginic acid, mannitol and fucans, some of them sulfated and with anticoagulant, anti-inflammatory and antitumor activities [6].
With the aim of using compounds as mentioned above for therapeutic and pharmaceutical purposes, several groups have linked the demonstrated bioactivities of brown algae to the presence of different chemical compounds such as polyphenols [7]. There is great interest in phenolic compounds due to their antioxidant properties and their possible implications in cardiovascular disease, cancer cell inhibition, cholesterol, among others [8]. Several articles report on fast, reproducible and selective techniques for the extraction of phenolic compounds from species of the genus Sargassum. Some of them comprise Ultrasound-Assisted Extraction (AUE), Microwave Assisted Extraction (MAE), Supercritical Fluid Extraction (SFE), Pressurized Liquid Extraction (PLE), Enzyme Assisted Extraction (EAE), among others [9]. In Cuba, extraction processes implemented for these purposes are in line with more traditional procedures, such as solid-liquid extraction (SLE) methods employing stainless-steel reactors adequate for the extraction of these metabolites, without using more modern equipment which implies higher production costs. Moreover, despite reports on phenolic compounds content in Sargassum sp. algae [10], there were not found techniques able to improve the extraction efficiency of total polyphenols in this particular genus with that traditional methods.
Previous studies run at the Center for Marine Bioproducts, in Havana, Cuba, showed that up to 0.23 mg/ g dry extract of phenolic compounds could be obtained from a 50 % ethanol hydroalcoholic extract [11]. Therefore, this work was aimed to optimize the extraction conditions of this method, to improve yields of total polyphenols in the extract, using brown seaweed Sargassum fluitans Børgesen (Børgesen) drifts to Cuban shores as starting material.
MATERIALS AND METHODS
Collection
The specimens of S. fluitans were collected in February 2015 from drifts on Guanabo Beach (23º10’44 “N and -82º07’01” W), Havana, Cuba, identified by Beatriz Martínez Daranas, Ph.D. (Center for Marine Research). They were stored in the collection of the National Aquarium of Cuba, labeled as HANC AC001. The algae were washed with tap water to remove salts, sand and other debris, and subsequently dried (15 % maximum humidity) in a ventilation oven at 50-60 °C until constant weight. Later on, they were milled in a hammer mill to a particle size of less than 6-mm fragments and stored in black polyethylene containers on shelf under controlled humidity (65-70 %) and temperature (28-30 ºC) conditions.
Preparation of extracts
Extractions were made by applying hydroalcoholic solutions (20, 35 and 50 % ethanol concentration) to algae material at a 10:1 v/w ratio. Each test was run with 10 g of dry and milled algae, which was mixed with 100 mL of hydroalcoholic solution and placed on a water bath at 50 °C, under constant stirring at 800 rpm for 90, 120 or 140 min. Afterwards, the resulting extract was cooled to 25 °C and collected by filtration through qualitative Whatman filter paper, 180 mm in diameter (Whatman, USA), and stored in amber flasks at 4 °C until analysis.
Determination of total polyphenols
Total polyphenols concentrations were determined from extracts by interpolating the absorbance of samples in the standard curve obtained from increasing concentrations (0.1-0.6 mg/mL) of pyrogallol reagent (BDH, USA). Standard curve determinations were made by the Folin-Ciocalteu spectrophotometric method [12]. Absorbance of samples and the standard was measured at 760 nm with a UV-Vis spectrophotometer (Shimadzu UV-1201, Shimadzu, Japan). The results were expressed as milligrams of pyrogallol (standard) per gram of dry extract.
Experimental design
A complete 32 factorial design with replicates in the center was performed to optimize and evaluate the dependence of the content of total polyphenols as response variable (dependent variable). It was analyzed as a function of variation of extraction time (90, 120 and 140 min) and ethanol concentration (20, 35 and 50 %, v/v) as independent variables.
Then, the quadratic model regression equation was derived for each independent variable, equaled to zero, and each relative or local optimum value was calculated. Additionally, the response variable was correlated with independent variables by adjusting the prediction of the response variable by using a quadratic polynomial equation, which general formula is as follows:
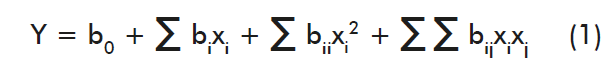
Where: Y is the dependent variable (response variable); Xi is the independent variable influencing the response variable; b0 is the linear coefficient; bii is the quadratic coefficient and bij is the interaction coefficient [13].
Subsequently, the second derivative was evaluated with optimal values, verifying that a local maximum of the function for the study area was attained. Then, the regression curve was obtained with the significant coefficients from the values.
Statistical analysis
The response variable (total polyphenol concentration) was analyzed by the response surface methodology. The experimental design, the statistical analysis of data and the regression model were generated with the STATISTICA software (version 4.0; StatSoft Inc., USA).
RESULTS
In order to establish the optimal conditions for improved yields of phenolic compounds extracted from Sargassum fluitans Børgesen (Børgesen) with ethanol, a complete 32 factorial design was applied with extraction time (90, 120 and 140 min) and ethanol concentration (20, 35 and 50 %) as independent variables. As shown in table 1, extraction values ranged 7.01-8.63 despite the variant used.
Total polyphenols concentrations were estimated from a Pyrogallol calibration standard curve, following the formula: y = 7.9449x + 0.0062 (R2 = 0.9975).
The regression model coefficient obtained for this equation corresponds to variables having significant effects on the concentration of total polyphenols. It is observed that a high ethanol concentration in the extraction solution negatively influences on the content of polyphenols, and, conversely, a decrease in ethanol concentration leads to an increase in the total polyphenol content. On the other hand, extraction time does not have significant effects on the yields, determining that this variable was not further considered.
Regarding multiple interactions (ethanol concentration for each extraction time, (ethanol concentration)2 and (ethanol concentration)2), they showed an inverse dependence, their decrease leading to an increase in polyphenols content. Based on these results, an adjusted polynomial was generated as follows:
![]()
Where: t: Extraction time; and [EtOH]: Ethanol concentration.
The coefficients of the regression equation forming the gradient vector provided the clues to find optimal conditions. Variance analysis provided a determination coefficient (R2) of 0.9719, with an optimal adjustment of the model for the independent variable in the range evaluated, as expected [14].
Subsequently, the response and contour surfaces were obtained (Figure), delineating the various effects independent variables have on the dependent variable. As shown in figure A, the response surface of total polyphenols content indicates that maximum values of total polyphenols can be obtained below and close to the center of the plane as a function of ethanol concentration and extraction time, respectively. In the case of the contour surface chart (Figure B), relating the ethanol concentration and extraction time to total polyphenols content, it depicted to what levels the operating point can move without leaving the optimum zone during extraction. This guarantees obtaining maximum concentration values of total polyphenols while adapting operational conditions to specific experimental settings.
Considering all these results, an optimal combination of both hydroethanol solvent concentration and extraction time was set for the extraction of phenolic compounds from S. fluitans, by adding 17.75 % ethanol hydroalcoholic solution (10:1 mL/g of dry seaweed) and incubation at 50 ºC under stirring at 800 rpm for 123.5 min. Hence, the function optimum combination was experimentally confirmed in three replicates (Table 2).
DISCUSSION
The extraction method by maceration with agitation and heat has proven effective and time saving to obtain phenolic compounds from seaweeds [15]. Nevertheless, few studies refer to its use with S. fluitans as starting material. Particularly, the time for extraction is an important factor to obtain polyphenols, with an excessive time regarded as detrimental. In fact, and according to the Fick’s diffusion law, a final equilibrium is expected to be achieved at longer contact times between the solute of the solid matrix and the solvent during the extraction process [16]. This dynamics is fundamental to take advantage of the great potential of marine organisms as a significant source of secondary metabolites, with more complex structures as biomolecules which cannot be found in terrestrial organisms [17].
In this regard, polyphenols are among those molecules of therapeutic potential, with many properties derived from their radical scavenging activity as antioxidants, which can be isolated from seaweeds. For instance, Nakai et al. [18] identified and isolated bifuhalol (Phlorotannins) from a Sargassum species. But other groups have reported yields of total polyphenols, when using the maceration and heat extraction method, lower than the ones obtained with our process [19-21].
It was further observed that the higher the ethanol concentration, the lower the yields of total phenolic compounds extracted, with ethanol concentration as a significant variable during the process. This effect could be possibly related to the overall polarity of the compounds present in this species and their interaction or binding to glycosylated groups [22]. In other results, Valdés Iglesias et al. [11] showed that maceration with occasional agitation of extracts of S. fluitans rendered as few as 0.23 mg/g of dry seaweed material. Here we have shown that with optimized conditions and after the effective factorial engineering of the variables involved, yields can be readily improved to up to 8.66 mg/g of S. fluitans dry material. It is considered that the effective combination of incubation time and ethanol concentration at the assay temperature (50 ºC) facilitates the expansion of components of the vegetal seaweed cell wall and the passage of secondary metabolites into the extraction solution, this process accelerated by agitation [23].
Our optimized extraction conditions gain relevance when facing industrial scale-up, since it uses low costs reagents (ethanol) and standard equipment, and the process is environmental friendly (low polluting gas emission). Moreover, the process employs natural materials regarded as waste (i.e., seaweeds seasonal banks) as raw material, rich in bioactive compounds, aiding on the national production of those compounds for its application in the pharmaceutical industry.
In summary, a relatively simple, inexpensive and scalable extraction process was implemented and optimized, for the extraction of phenolic compounds from the seaweed S. fluitans. It combines the maceration with agitation and heat method with adjusted concentrations of ethanol, providing a significant source of total polyphenols which can be used either for practical application as for characterization studies of the seaweed species arriving to Cuban shores.
REFERENCES
1. Nuñez R, Garateix A, Laguna A, Fernández MD, Ortiz E, Llanio M, et al. Caribbean marine biodiversity as a source of new compounds of biomedical and others industrial applications. Pharmacologyonline. 2006;3:111-9.
2. Liu H, Gu L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J Agric Food Chem. 2012;60(5):1326-34.
3. Yende SR, Harle UN, Chaugule BB. Therapeutic potential and health benefits of Sargassum species. Pharmacognosy Rev. 2014;8(15):1-7.
4. Sánchez Camargo AP, Montero L, Barranco A, Cifuentes A, Ibáñez E, Herrero M. Empleo de procesos verdes para la obtención de polifenoles de la macroalga Sargassum muticum recolectada en diferentes localizaciones de la costa europea. I Jornadas Científicas del CIAL Comunicaciones congresos, Madrid, España. 2014 Jun 5 (cited: 2016 Oct 17). Available from: http://hdl.handle.net/10261/109527.
5. Parys S, Rosenbaum A, Kehraus S, Reher G, Glombitza KW, König GM. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J Nat Prod. 2007;70:1865-70.
6. Medeiros VP, Queiroz LS, Abreu LRD, Queiroz KCS. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed Pharmacother. 2008;62(5):303-7.
7. Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch Pharm Res. 2004;27(2):194-8.
8. Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003;61(9):1273-5.
9. Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300-12.
10. Thillaikkannu T, Balamurugan M, Sivakumar K. Screening of phycochemical constituents qualitatively and quantitatively certain seaweeds from gulf of mannar biosphere reserve. Int Res J Pharm. 2012;3(7):261-5.
11. Valdés Iglesias OR, Hernández Y, Garateix A, Fernández MD, Ortiz E, García T, et al. Informe Final del Proyecto No. 10010015 de la Agencia de Medio Ambiente “Uso sostenible de las algas marinas del archipiélago cubano con fines nutracéuticos”. 2009 [cited: 2016 Oct 17]. Available from: http://repositorio.geotech.cu/jspui/handle/1234/179.
12. British Pharmacopoeia. Vol IV. (Appendix XI M) Tannins in Herbal Drugs. London: The Stationery Office; 2010 [cited 2016 Oct 17]. Available from: www.pharmacopoeia.co.uk.
13. Jiménez J, Guardia-Puebla Y, Cisneros-Ortiz ME, Morgan-Sagastume JM, Guerra G, Noyola A. Optimization of the specific methanogenic activity during the anaerobic co-digestion of pig manure and rice straw, using industrial clay residues as inorganic additive. Chem Eng J. 2015;259:703-14.
14. Wang Y, Wu H. Improvement of biodiesel production by lipozyme TL IM catalyzed methanolysis using response surface methodology and acyl migration enhancer. Bioresour Technol. 2008;99:7232-7.
15. Oyesiku OO, Egunyomi A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr J Biotechnol. 2014;13(10):1188-93.
16. Pinelo M, Ruiz-Rodríguez A, Sineiro J, Señoráns FJ, Reglero G, Núñez MJ. Supercritical fluid and solid–liquid extraction of phenolic antioxidants from grape pomace: a comparative study. Eur Food Res Technol. 2007;226:199-205.
17. Srivastava N, Saurav K, Mohanasrinivasan V, Kannabiran K, Singh M. Antibacterial potential of macroalgae collected from the Madappam coast, India. Br J Pharmacol Toxicol. 2010;1(2):72-6.
18. Nakai M, Kageyama N, Nakahara K, Miki W. Phlorotannins as radical scavengers from the extract of Sargassum ringgoldianum. Mar Biotechnol. 2006;8:409-14.
19. Chandini SK, Suresh P, Bhaskar VN. Seaweeds as source of nutritionally beneficial compounds-A review. J Food Sci Technol. 2008;45:1-13.
20. Echavarría B, Franco AS, Martínez AM. Evaluación de la actividad antioxidante y determinación del contenido de compuestos fenólicos en extractos de macroalgas del caribe colombiano. VITAE. 2009;16(1):126-31.
21. Quitral VR, Morales CG, Sepúlveda ML, Schwartz MM. Nutritional and health properties of seaweeds and its potential as a functional ingredient. Rev Chil Nutr. 2012;39(4):196-202.
22. Se-Kwon K, Himaya SWA. Medicinal effect of pholotannins rom marine brown algae. Adv Food Nutr Res. 2011;64(8):97-109.
23. Miranda M, Cuellar A. Farmacognosia y Productos Naturales. La Habana: Editorial Félix Varela; 2001.
Received in October, 2016.
Accepted in May, 2017.
Richard Gutiérrez. Departamento de Química, Instituto de Ciencias del Mar (ICIMAR) Calle Loma #14 e/ 35 y 37, Nuevo Vedado, Plaza de la Revolución, CP10600, La Habana, Cuba. E-mail: richard@cebimar.cu; gutierrezcuba65@gmail.com.














