Introduction
Nowadays, the use of functional foods has increased, which are potentially healthy products, including any food or food ingredient, modified or not, that can provide a beneficial effect on health, and traditional nutrients (Gibson and Roberfroid 1995). This is due, among other causes, to the fact that the population has greater concern for their health and increase of their life expectancy, which has led to the search for new compounds, secure and innocuous, that reduce the risk of diseases.
Disorders in lipid metabolism are one of the most frequent diseases worldwide. For this reason, producers and consumers look for healthy products for human and animal nutrition, which influence on reducing the effects of cholesterol and triglycerides, among which we can mention probiotics and prebiotics (Sharma y Puri 2015 y Yang et al. 2018).
Prebiotics are defined as substrates that are selectively used by host microorganisms, conferring health benefits (Gibson et al. 2017). Different researches showed the prebiotic effect of agavins obtained from different Agave species (García-Vieyra et al. 2014, García-Curbelo et al. 2015a and Huazano-García and López 2015). The objective of this research was to evaluate the effects of agavins of Agave fourcroydes on indicators of lipid metabolism growing pigs.
Materials and Methods
Prebiotic. Agavins of Agave fourcroydes were used, constituted by oligosaccharides with a degree of polymerization < 10, with bonds of type β (2-1), β (2-6), ramifications and neoseries (García-Curbelo et al. 2015b).
Animals, management and treatments. One hundred twenty pigs (Yorkshire-Landrace x L35 hybrid) of 33 d old and initial mean body weight of 8.0 ± 0.95 kg were used in the experiment. At the beginning of the experiment, pigs were randomly distributed into three treatments using a randomized block design, with eight replicates per treatment and five pigs per pen. The experiment lasted 75 d and was developed in the experimental pig unit of the Institute of Animal Science, located in Mayabeque province, Cuba.
Three treatments were used, which consisted of a standard diet of maize and soybeans (STD) and the addition of 0.25 % of agavins of Agave fourcroydes (AF-C 0.25 %) and 0.50 % of agavins of Agave fourcroydes (AF-C 0.50 %) to the standard diet, respectively. The diet was prepared according to the requirements of the National Research Council (1998). No antibiotics or coccidiostats were included. During the entire experimental period, pigs had ad libitum access to food and water.
Determinations. Liveweights of the animals were determined at the beginning and end of the experiment. At the end of the experimental stage, a pig was randomly selected from each pen and blood samples were taken from the jugular vein. Samples were centrifuged at 2000 xg at 4 °C for 30 min. and serum was separated, and stored at -20 °C until their analysis. Cholesterol, high-density lipoprotein-cholesterol (HDLc), triglycerides and total lipids were measured using commercial diagnostic enzymatic kits (Centro de Radioisótopos, Cuba).
Very low-density lipoprotein-cholesterol (VLDLc) was estimated by the equation VLDLc=TG/5, and low-density lipoprotein (LDLc) by the equation
Statistical analysis. For the analysis, the statistical package INFOSTAT (Di Rienzo et al. 2012) was used. For the statistical treatment of data, analysis of variance was performed and comparison test of Duncan (1955) was used for detecting significant differences.
Results and Discussion
Table 1 shows that there was no effect of agavins of Agave fourcroydes on liveweight of animals.
Figure 1 shows the reduction of serum cholesterol levels with the addition of the prebiotic, achieving a decrease of this indicator in the animals treated with agavins (0.25 % and 0.50 %), with a reduction of 26 % and 20 %, respectively , in relation to the standard diet.

Figure 1 Concentration of serum cholesterol in growing pigs with the addition of agavins of A. fourcroydes
Prebiotics can regulate lipid metabolism. Different studies have proposed possible mechanisms of modulation by means of short chain fatty acids, intestinal peptides or enzyme-regulating genes in the liver (Delzenne et al. 2002 and Liu et al. 2017). The results of this study showed that the intake of agavins did not increase liveweight, but decreased serum cholesterol levels in pigs. This could be related to the structure of these compounds, by presenting β-type bonds. These are not degraded by digestive enzymes of the host in the upper parts of the gastrointestinal tract, and reach the large intestine, where they are fermented by the beneficial microbiota. This causes an increase in the production of short-chain organic acids and a decrease of pH (unpublished data). The production of propionic acid by fermentation participates in the decrease of cholesterol, since it causes inhibition of the hepatic enzyme 3-hydroxy-3- methyl-glutaryl-CoA reductase (HMG-CoA reductase), which regulates this synthetic metabolic pathway ( Jackson and Lovegrove 2012).
The hypocholesterolemic activity was obtained by Gallaher et al. (2000), finding similar effects in rats with the administration of glucomannans and chitosan as prebiotic sources. It was also obtained by García-Curbelo et al. (2015a), with the use of agavins of Agave fourcroydes in mice. However, other studies do not report changes in lipid metabolism, when using prebiotic. Research conducted by Grela et al. (2014), with the use of chicory inulin as an additive in pigs, did not achieve changes in cholesterol levels. Studies in diabetic patients with fructooligosaccharides did not modify lipid metabolism either (Luo et al. 2000).
Table 2 shows the effect on triglycerides and lipoproteins related to cholesterol transport. Only low- density lipoproteins decreased in the groups treated with agavins, being the group of AF-C 0.25 % the one that achieved the greatest reduction, with 40.66 % with respect to the untreated group.
Table 2 Concentration triglycerides and lipoproteins related to transport of cholesterol in growing pigs with the addition of agavins of A. fourcroydes.
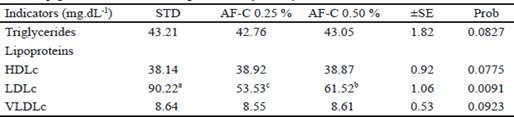
a,b,c Values with different subscript in the same line are significantly different (P < 0.05).
The decrease of cholesterol with the use of agavins was related to the reduction of bad cholesterol (LDLc), which is one of the lipoproteins responsible for its transfer to different peripheral and storage tissues, including adipose tissue. However, it did not influence on the concentrations of triglycerides (VLDLc), nor in high-density lipoproteins (HDLc), responsible for taking cholesterol to the liver for its degradation. Studies conducted by Mortensen et al. (2002), with the use of long chain fructans in mice, also found a reduction of cholesterol and low density lipoproteins. Liong et al. (2007), with a symbiotic with lactobacilli and fructooligosaccharides, obtained a reduction of total cholesterol in plasma, triglycerides, and LDL cholesterol in pigs. However, this paper does not evidence the hypothesis proposed by Delzenne and Kok (2001), related to the reduction of novo lipogenesis in the liver by reducing the activity of all lipogenic enzymes in rats fed fructans.
The concentration of total lipids also decreased with the addition of agavins in the diets (figure 2), being higher in the group AF-C 0.25 %, with 22.61 % reduction with respect to the control.
The reduction of total lipids was related to the reduction of cholesterol with the use of agavins and their influence on the decrease of LDLc. The effect of prebiotics on metabolism of lipids requires further research, since there are contradictory answers in the scientific literature. This could be related to different factors, such as optimum dose of products, frequency and duration of treatment, physiological state, species, feeding, age, sex, and some other aspects.

Figure 2 Concentration of total lipids in growing pigs with the addition of agavins of A. fourcroydes.
It is concluded that the inclusion of agavins of Agave fourcroydes in the diet of pigs produced modifications in lipid metabolism, related to the decrease of total cholesterol, LDLc and total lipids.











 text in
text in 



