Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Centro Azúcar
versión On-line ISSN 2223-4861
cen. az. vol.45 no.3 Santa Clara jul.-set. 2018
ARTICULO
Use of native cellulolytic enzymes from ecuador, in the enzymatic hydrolysis for the ethanol production
Uso de enzimas celulolíticas nativas de ecuador, en la hidrólisis enzimática para la producción de etanol
Jonathan Villavicencio Montoya1*, Carmen Amelia Salvador Pinos2, Fernando Batallas Merino1, Amaury Pérez Martínez3, Layanis Mesa Garriga4 y Erenio González Suárez1
1 Departamento Ingeniería Química. Facultad de Química y Farmacia. Universidad Central "Marta Abreu" de Las Villas. Carretera a Camajuaní Km 5 ½, Santa Clara, Villa Clara, Cuba.
2 Facultad de Ciencias Médicas. Universidad Central del Ecuador, Av. Universitaria, Quito 170129, Ecuador.
3 Departamento de Ciencias de la Tierra. Universidad Estatal Amazónica. Troncal Amazónica E45, Puyo, Ecuador.
4 Departamento de Bioproductos. Sede Central. Corporación Colombiana de Investigación Agropecuaria (CORPOICA). Km 14 Vía Mosquera - Bogotá, Colombia.
*Autor para la correspondencia: Jonathan Villavicencio, Email: Jfvm_1993@hotmail.com
ABSTRACT
This study shows the use of cellulolytic enzymes Bacillus sp. in enzymatic hydrolysis process and their use in the production of second-generation ethanol. For doing this, The Plackett-Burman method was used to discard variables that do not influence the enzymatic hydrolysis process, and subsequently to adjust the model using the factorial optimization design Box-Hunter 25-2. Once the conditions of the experiments in the enzymatic hydrolysis process were optimized, the best testwas selected considering the highest glucose yield per 100g bagasse. The fermentative stage was also analyzed, resulting in a possibility of producing 1 liter of second-generation ethanol per 12.83kg bagasse and 77.9 liters per ton of bagasse.
Keywords: Enzyme cocktail; enzyme production; second-generation ethanol.
RESUMEN
El presente estudio muestra el uso de enzimas celulolíticas de Bacillus sp en el proceso de hidrólisis enzimática y su empleo en la producción de etanol de segunda generación.
Se emplea el método de Plackett-Bürman, para descartar variables que no influyen en el proceso de hidrólisis enzimática, para así posteriormente ajustar el modelo y utilizar el diseño de optimización factorial de Box-Hunter25-2. Una vez optimizadas las condiciones de los experimentos en el proceso de hidrólisis enzimática, se seleccionó el mejor ensayo considerando el rendimiento más alto de glucosa por 100g de bagazo, y se analizó la etapa fermentativa, obteniendo como resultado que se producirá 1 litro de etanol de segunda generación por cada 12,83kg de bagazo y 77,9 litros por cada tonelada de bagazo.
Palabras clave: cocktail enzimático; producción de enzimas; etanol.
INTRODUCTION
Lignocellulosic waste material as an energy resource has become more important, due to the fact that some energy crises have been generated globally as a result of fluctuations in oil prices over the last fifty years (González et al., 2008).
This circumstance has led to the search for more suitable raw material for the production of fuels as an alternative to traditional sources, originated from fossil materials, coupled with the environmental deterioration, caused by toxic compounds generated during their combustion (Gualteros, 2011). Therefore, the search and use of new resources and chemicals is required and this is where biomass and particularly lignocellulosic biomass, is revealed as a very promising source of renewable energy and chemicals generation in the world (Alvarez et al., 2012).
The ethanol produced by sources of lignocellulosic material has become an alternative use to the waste generated in the sugarcane industry, being this process with high accessibility to the raw material, since no significant advantage to bagasse is given, and the cost thereof is relatively low (Guarnizo et al., 2009).
One of the main challenges of the marketing process for obtaining ethanol from lignocellulosic materials is to make the process economically sustainable (Mesa, 2010).
To evaluate the effectiveness of new microbial cellulolytic enzymes in respect to glucose production from vegetable waste, an ideal way is experimentation using pilot schemes in which the combination of the tests of an experiment is proposed.
The efforts in developing methods to find optimal conditions, have a contribution for the work of (Box and Hunter, 1961), in which all the factors of the study are varied without having to make all possible experiments, called fractional factorial design; achieving the main objectives of the experimental design of reducing the number of tests and obtaining more information.
Considering that the production of microbial enzyme cocktail produced at laboratory scale can be carried on an industrial scale, by understanding the conditions required by the hydrolysis process; some research are aimed at finding new microorganisms within the existing diversity or even create new microorganisms at laboratory scale. In this research a microorganism that has been collected in a craft factory of panela (It is a sweetener derived from the sugar cane juice) production in Balzapamba is used, which has shown interesting cellulolytic activities (Salvador, 2017). From this analysis, the research has set the overall aim: to determine the best conditions of enzymatic hydrolysis of Bacillus bacteria, native to Ecuador, that is going to be used in the production of second-generation ethanol.
MATERIALS AND METHODS
2.1. Pretreatment of lignocellulosic material
2.1.1. Raw material
Sugar cane bagasse (60% w/w) was collected in Puyo-Ecuador. Then it was triturated until a size of 1.5 mm for its use in the experiments. The composition of the material in relation to the percentage of dry matter was glucan, 49.0%; xylan, 15.6%; 27.24% lignin.
2.1.2. Acid hydrolysis
This treatment was applied to 500 grams of bagasse, with 1.25% sulfuric acid (w/w) and a relation of bagasse and sulfuric acid 1:10. This was put in the autoclave for 40 minutes at 134 ºC and 2 atm of pressures. After this, the liquor was collected, and bagasse was pretreated with water in a proportion 1:1, then filtered and moved to the next pretreatment.
2.1.3. Hydrolysis basic-organosolv
30% ethanol and 7% NaOH, dry fiber concentration was added to the filtrate. The NaOH bagasse ratio is 1:7 and was placed in the autoclave at 175 ºC for 90 minutes. The pretreated solid was washed with water to remove the ethanol and alkali, to pass to a drying stage of four hours at 40 ºC, later the sample was analyzed to see the remnants of glucose, xylose and lignin content (Sidiras and Salapa, 2015).
2.2. Enzymatic hydrolysis of bagasse
According to the qualitative and quantitative tests, two microorganisms were chosen and evaluated for the enzymatic hydrolysis of bagasse, the hydrolysis was performed with 5% pretreated bagasse, 1 ml of citrate buffer (pH 4.8) and 0.1 g of Tween 80 surfactant. The enzymatic crude was obtained by filtering the medium in which the microorganism had grown. 2 ml of enzymatic cocktail were placed and measurements of the established conditions were made according to (Salvador et al., 2018).
2.3. Experimental design
To achieve these objectives, the possibility of combining the Plackett-Bürman method (González et al., 1986) of multivariable systems with partial recesses for polynomials of the first degree of the Box-Hunter method (1961). The matrix for rotational designs was completed, taking full advantage of all the runs made and calculating a quadratic equation, as appropriate. The combination of the Plackett-Bürman method with that of Box-Hunter has already been applied in hydrolysis research with enzymes, obtaining satisfactory results (Salvador et al., 2018) that have served for the identification of some stages and systems of the process.
2.3.1. The method of Plackett-Bürman
The method of Plackett-Bürman (Plackett and Bürman, 1946) is based on a highly fractional factorial design that studies all the possible variables that affect the system, and manages to determine which the most influential ones are. It is considered an initial program to study processes that have a high number of variables and should continue research in the experimental region, after discarding non-influential variables, with a more rigorous plan, with the aim of finding a more appropriate model.
To apply the method, Isaacson (1970) recommends that additional experiments to estimate the standard error and the variance, due to experimental errors, interactions or quadratic effects; that is why false variables are included in the experimental plan.
2.3.2. Box-Hunter optimization design (Box and Hunter, 1961).
All the information provided by a 2nplan may not be of interest, since interactions of a higher order are usually negligible. In these cases, it is advisable to use the fractional plans, as they are useful:
a. when there are negligible interactions,
b. in the determination of significant variables,
c. in sequential investigations where the results lead to modify our experiences and
d. when the influences of several factors can be described by a single main effect.
With the new conditions that influence the experiment, we proceeded to measure glucose yield, taking into account the results of the Box-Hunter experimental design.
For the production of second-generation ethanol from the results obtained in glucose yield per 100g of bagasse, the experimental results of the fermentative stage (Hydrolysis and separate fermentation, HFS) and ethanol extraction were taken (Mesa, 2010).
RESULTS AND DISCUSSION
The best conditions of the enzymatic hydrolysis were determined starting from the experience reached by Salvador and collaborators (Salvador et al., 2018), including as response variables the following:
3.1. Dependent variables
Y1: the grams of glucose yields per 100 grams of raw material, in order to take advantage of the cellulose composition, consists of a polysaccharide formed by β-1,4 glycosidic bonds (Cuervo et al., 2009).
For the study, the following independent variables recommended by (Salvador et al., 2018) were considered:
3.2. Independent variables
X1: Temperature (35 ºC -50 ºC).
The enzymatic hydrolysis is carried out at the optimum temperature of the commercial enzyme, around 50 ° C, and decreases up to 35 ° C because it has been shown that these enzymes are more active in that range of temperatures (Ruegm and Tauke, 2004), (Baig et al., 2004), (Mesa, 2010).
X2: Units of filter paper (UFP) (:10-25 UFP/g)
A cellulose concentrate (10 UFP / g of dry base) has a positive effect on hydrolysis, to obtain better glucose yields. In addition, the literature indicates that an enzyme load of 25 UFP / g is the most indicated to achieve better glucose yields (Peñuela et al., 2007).
X3: Agitation, in rpm (150-200 rpm).
It has been described that enzymes work much better with agitation, projecting higher glucose yield results (Mesa, 2010).
X4: Enzymatic reaction time (15-24 hours).
Preliminary studies found good yields in a time of 24 hours (Lin et al., 2010). In addition, it is suggested that the best glucose yields are obtained in tests performed between 8 and 72 hours, having more emphasis on 24, and see how the glucose yield differs in a shorter time such as 15 hours.
X5: Solid state in percentages (5 and 8%).
According to theoretical studies, the hydrolysis is best executed in a semi-solid state (Zhang et al., 2017). Also according to a study carried out in the analytical laboratory of renewable energy procedures of the Central University of Ecuador, provided results of glucose yields using a solids load of 2% w / v. A load of solids was maintained between 6 and 10% to achieve high yields (Peñuela et al., 2007). Other authors confirm a very acceptable glucose yield using 5% w /v (Buaban et al., 2010) (Rodrígues et al., 2014).
X6: Use of Tween 80 surfactant, in percentages (0.1g and 0.2g).
The incorporation of commercial surfactants into the enzymatic hydrolysis stage has an effect on the increase of glucose yield up to about 20% (Mesa, 2010).
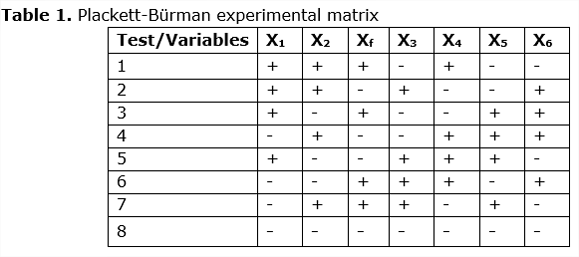
The experimental matrix of Plackett-Bürman that was proposed based on the analysis carried out for enzymatic hydrolysis with enzymatic cocktail Bacillus sp bacteria in order to determine the significance of each of the variables, is observed below:
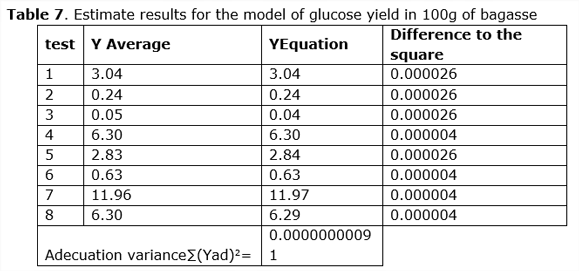
The results obtained in the planned experiment show that the best test is 7, which has 11.96 glucose yield per 100g of bagasse. Therefore, the conditions presented by the test for the bacteria, in relation to the factors involved for the degradation of the lignocellulosic material were the best (See Table 2).
The glucose yield coefficient for the factors studied is presented below in Table 3:
When implementing the design of Plackett-Bürman, by means of the coefficients of glucose yield, it is possible to observe the variables that are not significant in the experiment. This way discarding the conditions mentioned above, optimizing the enzymatic hydrolysis process.
The standard error is obtained by calculating the false estimated variables (equation 1), just as in the case of the real variables (Isaacson, 1970), thus:
The simplification of each effect is verified by comparing the tabulated value of the student's t to F/ numberof false variables and the calculation of the expression 2:
Then, if the calculated value is greater than the tabulated one, it means that the effect of the level variation of the independent variable really causes variations in the response parameter. And this is not due to experimental errors, which depends on the degree of significance of the variable, it is obtained as it is significant: P = 80, 85, 90, 95% and they are compared with the tabulated T values.
The E3 agitation is not significant because the amount of bagasse that enters the process, when mixed with the enzymatic cocktail of bacteria, forms a cake that makes agitation difficult, this saccharification begins as a semisolid hydrolysis. Table 4 shows that Ef (false variable) and E3 (agitation) are not significant for the experiment, the false variable is discarded.
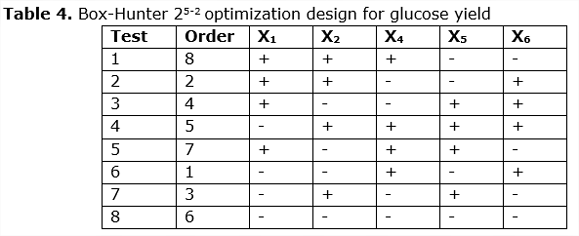
Once condition X3 (agitation) has been discarded, a fractional factorial design is carried out, taking into account the conditions that do influence the experiment.
3.3. Box-Hunter optimization design
Fractional factorial design 25-2) in the enzymatic hydrolysis with enzymatic cocktail of bacterium Bacillus sp, with conditions adjusted to the experimental data obtained in the laboratory are shown in Table 4:
Here two relationships of the type were established:
Then:
What generates the following mixing effects of the independent variables:
This makes it possible to ensure that the model for the estimate will have the terms corresponding to the independent variables, because the effects of the interactions are mixed with those of the independent terms.
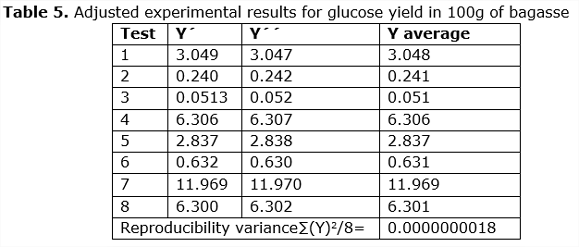
The data adjusted for glucose yield, after replication, by means of the fractionated optimization design, taking into account the factors that influenced the enzymatic hydrolysis process, are shown in Table 5.
By discarding non-influential conditions, the costs of the processes, thus obtaining an optimization in the production.
Once the conditions that influence the experiment are considered, the average between the experiment Y’ y Y’’is calculated, and then it is compared with the result obtained when solving the equation of Box-Hunter model, thus obtaining the experimental errors for each of the tests (See Tabla 5).
3.3.1. Box-Hunter equationmodel
With the experimental design, the reproduction variance = 0.0000000018 was calculated and with the comparison between the experiments and the estimated by the model, the adequacy test was determined by the Fisher 2 test at 8 = 0.0000000091 less than the tabulated Fisher (Perry and Chilton, 1975).
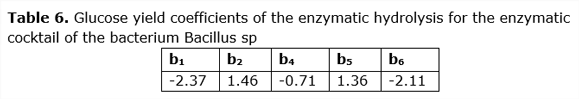
The glucose yield coefficients shown in Table 6 show the most influential variables in the experiment, these being: b1 (Temperature) and b6 (Tween 80) and when selecting the best test in terms of glucose yield (see Table 2), it can be concluded that the enzymatic cocktail of the bacterium Bacillus sp, produces a better glucose yield at a lower temperature and with less use of Tween 80.
In addition to the previous one, the remaining 24 possibilities of the design were explored with the help of the model obtained from Box-Hunter and it was determined that no other experiment would give better results.
3.4. Fermentative stage
3.4.1. Results of separate hydrolysis and fermentation (SHF).
The SHF fermentation step was carried out with the results of the test 7 which has the highest value in terms of glucose yield in 100g of bagasse. The test was carried out at 35 °C, with an enzymatic load of 25 UPF, at 200 rpm, with 6% solids and 0.1% Tween 80. The hydrolysis was carried out in a period of 15 hours and the fermentation in a period of 24 hours.
The glucose concentration values obtained in the selected test at 15 hours of enzymatic hydrolysis were 11.92 g per 100 g of bagasse, and based on the theoretical yield of 0.51 g of ethanol / g of glucose (Mesa, 2010), it was obtained that there is 6.08g of ethanol in 100g of bagasse. Then it can be concluded that 12.83kg of bagasse will be needed to produce 1 liter of ethanol, considering only the glucan fraction, and for each ton of bagasse, 77.9 liters of ethanol will be obtained.
CONCLUSIONS
1. The use of the enzymatic cocktail of native bacteria from Ecuador is an interesting proposal for the production of ethanol, since using enzymes produced with native bacteria could improve the costs of the enzymatic hydrolysis process.
2. The application of statistical designs will help to optimize the conditions used in laboratory experiments, which in turn will favor a future industrial scaling.
3. It is necessary to determine the production cost of the enzymatic cocktail of autochthonous bacteria to obtain ethanol, in this way the application in these results can be deepened.
4. It is important to study mixtures of autochthonous enzymes with commercial enzymes in the hydrolysis process, to analyze if glucose yields will improve.
REFERENCES
Alvarez, A., Salgado, R., Garcia, E., Dominguez , M., Granados, J., Aguirre, A., Aprovechamiento integral de los materiales lignocelulosicos., Revista iberoamericana de los polímeros, 2012, Vol. 162, No. 56, pp. 140-150.
Baig, M., Baig, L., I, B., & Yasmeen, M., Saccharification of banana agro-waste by cellulolytic enzymes., African Journal of Biotecnology, 2004, Vol. 3, No. 9, pp. 447-450.
Box, G., & Hunter, J., The key k-y Fractional factorial Desing., Techometrics, Vol. 3 No. 3, 1961, pp. 333-340.
Buaban, B., Inoue, H., Yano, S., Tanapongpipat, S., Ruanglek, V., Champreda, V., Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis., Journal of Bioscience and Bioengineering, Vol. 110, No. 1, 2010, pp. 18-25.
Cuervo, L., Folch, J.L., Quiroz, R.E., Lignocelulosa como fuente de azúcares para la producción de etanol., BioTecnología, Vol. 13, No. 3, 2009, pp.11-25.
González, A., Jiménez, I., Rodríguez , M., Restrepo, S., & Gómez, M., Biocombustible de segunda generación: Una mirada a la contribución de Universidad de los Andes. Revista de Ingeniería, Universidad de los Andes, Vol. 16, No. 11, 2008, pp.70-82.
González, E., Ramos, F., Ribot, A., & Peralta, L., Combinacion de los métodos de diseño experimental en la minimizacion de los ensayos de una investigación., Tecnología Química, Vol. 1, No. 6, 1986, pp.11-17.
Gualteros, J.M., Estudio prospectivo de la cadena productiva del biodiesel a partir de la palma africana en Colombia., Tesis presentada en opción al Grado Científico de Máster en Ingeniería Industrial, Universidad Nacional de Colombia, Bogotá, Colombia, 2011.
Guarnizo, A., Martinez, P., & Hoover , A., Pretratamientos de la celulosa y biomasa para la sacarificación., Scientia et Technica, Año XV, Vol. 2, No. 42, 2009, pp. 284-289.
Isaacson, W., Statistical Analysis for multivariable Systems Chemical Engineering., Vol. 29, 1970, pp. 67-74.
Lin, L., Yan, R., Liu, Y., & Jiang, W., In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: Cellulose, hemicellulose and lignin.,Bioresource Technology, Vol. 101, No.21, 2010, pp.8217–8223.
Mesa, L., Estrategia investigativa para la tecnología de obtención de etanol y coproductos del bagazo de la caña de azúcar., Tesis presentada en opción al Grado Científico de Doctor en Ciencias Técnicas, Especialidad Ingeniería Química en la Universidad Central Marta Abreu de Las Villas, Cuba, 2010.
Peñuela, M., Nascimiento, J., Becerra, M., & Pereira N., Enzymatic hydrolysis optmization to ethanol production by Simultaneus Saccharification and Fermentation., Biochem Biotechnol, Vol. CXXXVII, No. 212,2007, pp. 141-154.
Perry, R., & Chilton, C., Chemical Engineers Handbook., Advisory Board, McGraw-Hill, 1975, pp. 40-41.
Plackett, R., & Bürman, J., The design of optimum multifactorial experiments., Biometrika, Vol. XXXIII, No. 9, 1946, pp. 305-325.
Rodrigues, A.C., Felby, C., & Gama, M., Cellulase stability, adsorption/desorption profiles and recycling during successive cycles of hydrolysis and fermentation of wheat straw., Bioresource Technology, Vol. 156, 2014, pp.163–169.
Ruegm, M., & Tauke, L., Actividad de las celulasas de hongos aislados de suelos de la estación Ecológica de Sao Paulo, Brasil., Journal of Botany, Vol. 216, No. 19, 2004, pp. 324-331.
Salvador, C., Microorganismos con actividades celulíticas en los Andes de Ecuador., Seminario de actualización de investigación., Quito, Pichincha, Ecuador, 2017.
Salvador, C.A., Villavicencio, J., Batallas, F., Mesa, L., & González, E., Assessment of the best operating conditions in the enzymatic hydrolysis of pretreated bagasse for bagasse ethanol., MOL2NET, International Conference Series on Multidisciplinary Sciences, Puyo, Ecuador, 2018, pp. 55-63.
Dimitrios, K., Sidiras, Y., Ioanna, S.. Salapa, I., Organosolv pretreatment as a major step of lignocellulosic biomass refining.,Engineering Conferences International.ECI Digital Archives. Biorefinery I: Chemicals and Materials From Thermo-Chemical Biomass Conversion and Related.Processes, Crete, Grecia, 2015, pp. 17-37.
Zhang, H., Ye, G., Wei, Y., Li, X., Zhang, A., & Xie, J., Enhanced enzymatic hydrolysis of sugarcane bagasse with ferric chloride pretreatment and surfactant., Bioresource Technology, Vol. 229, 2017, pp.96-103.
Recibido: Marzo 5, 2018
Revisado: Abril 5, 2018
Aceptado: Mayo 8, 2018